Alpha-emitters can maximise tumour-cell destruction while minimising damage to surrounding cells.
External-beam radiation therapy is used routinely to treat many different types of cancerous tumours, delivering a targeted dose of radiation to cancer cells while sparing surrounding healthy tissue as much as possible. While there have been dramatic improvements in the control of patient and tumour dose during recent years, many challenges persist. These include side effects such as depressed immunity, which makes patients susceptible to post-treatment infections, and an increase in secondary cancers.
An alternative approach involves delivering a therapeutic radiation dose to tumour cells selectively through a strategy similar to that for molecular imaging: therapeutic isotopes are incorporated into complex pharmaceuticals for specific, targeted delivery of a potent radiation dose directly to cancerous cells. This approach has been recognised since the time of Madame Curie, but even after a century of development, this application remains woefully unoptimised.
To study the full potential of radionuclide therapy, the medical research community is increasingly demanding therapeutic alpha- and beta-emitting isotopes to treat advanced metastatic cancer and other diffuse diseases. Such therapeutic isotopes are changing the cancer-treatment landscape, yet lack of availability and cost are significantly affecting further research and development.
Targeted radionuclide therapy
Targeted radionuclide therapy (TRT) involves the injection of particle-emitting radionuclides appended to a biological targeting molecule, which direct a lethal dose of radiation to a specific site within the body. The short range and highly cytotoxic nature of alpha and beta particles destroys small, diffuse and post-operative residual tumours while minimising damage to healthy tissue. TRT’s strength lies in the diversity and adaptability of both isotopes and targeting molecules, which include monoclonal antibodies, antibody fragments, nanoparticles, and small peptides and molecules. Because this allows an optimal delivery regimen to be developed for each application, TRT isotopes are generating significant interest internationally.
Within the Life Sciences Division of TRIUMF in Vancouver, Canada, TRT is now an active research effort. The goal is to exploit TRIUMF’s production and radiochemistry capabilities to enable fundamental and applied research with a spectrum of isotopes across different disciplines. In the near-to-medium term, TRIUMF plans to develop platform technologies to enable accelerator-based radiometallic isotope production and applications beyond the current state-of-the-art. Access to a host of metallic isotopes will allow TRIUMF to leverage its radiochemistry expertise to demonstrate the synthesis of novel radiopharmaceuticals, including TRT drugs.
Alpha therapy in sight

Targeted alpha therapy (TAT) is a type of TRT that exploits the high linear-energy transfer of alpha particles (figure 1) to maximise tumour-cell destruction while minimising damage to surrounding cells. As such, TAT has tremendous potential to become a very powerful, selective tool for personalised cancer treatments. To fulfil its promise, however, TAT relies heavily on new developments in isotope production. It also demands organic, bioinorganic and organometallic synthesis techniques to create new molecular probes, and novel techniques to address the stability of metal complexes in vivo.
Several promising alpha-emitting radionuclides are currently under consideration worldwide – including 149Tb, 211At, 212Bi, 212Pb, 213Bi, 223Ra, 225Ac, 226Th and 230U – and very promising results have already emerged from clinical and pre-clinical studies of TAT agents. Progress at several laboratories is fuelling great optimism in the medical community. For example, the US Food and Drug Administration recently approved the use of the alpha emitter 223RaCl2 (registered under the trademark Xofigo) for pain relief from bone metastases, and several other TAT drugs are in the clinical-trial pipeline.
Securing a constant supply of clinically relevant amounts of alpha-emitting radionuclides remains a challenge, since their production requires high-Z targets and a complex infrastructure. “Generator systems” are a convenient source of TAT isotopes: for example, 225Ac (which has a half-life of 9.92 days) can be harvested as a decay product of 229Th. Because the global quantity of 229Th is not being replenished and the 229Th/225Ac generator can only be eluted every nine weeks, annual worldwide production is limited to approximately 1.7 curies. Several alternative strategies are therefore being proposed to produce such isotopes directly.
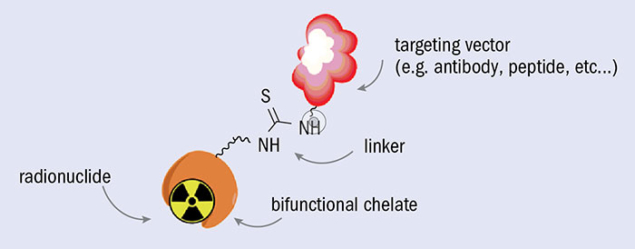
Image credit: Caterina F Ramogida.
TAT radionuclides must be carefully processed before being used in medical applications. They first must be isolated with high radio-chemical purity from the target material, which can be achieved using classical chemical procedures such as ion exchange, extraction and precipitation. Purified TAT radionuclides are then attached to biomolecule targeting vectors via a bifunctional chelator, which connects the biomolecule with a radionuclide complex (figure 2). The stability of compounds containing alpha-emitting radionuclides is a challenge because after decay most of the daughter isotopes are radioactive elements that no longer remain chelated. Moreover, the radioactive daughters can accumulate and cause unwanted toxicity in healthy organs, especially those involved in excretion such as the liver and kidneys. These issues have driven demand for a more robust and stable chelation system and/or encapsulation methods that contribute to an optimised pharmacokinetic profile with rapid cell internalisation. By doing so, the hope is to keep radioactive daughter nuclei proximal to the original decay site and thus close to the targeted tissue.
Several clinical trials with alpha-emitting radionuclides – including 225Ac (phase II trial) and 213Bi (phase III) – are under way around the world, based on the standard chelation approach. Despite the challenges involved, these trials are already showing extremely high promise and superiority over existing beta-emitting radionuclides. Further research is therefore warranted to investigate and optimise various production strategies designed to make TAT a viable clinical modality. The TAT isotope 225Ac has demonstrated particularly high potential in recent years because its half-life correlates well with the biological half-lives of intact antibodies, and its multiple alpha-emitting daughters enhance the therapeutic effect. 225Ac also can be used as a parent radionuclide for a 225Ac/213Bi generator system.
TRIUMF’s strategy
TRIUMF has extensive expertise in all aspects of the production of medical isotopes, including the development of high-powered targets for large-scale production and expertise in isotope-production simulations with its existing Monte Carlo code FLUKA and the new Geant4. TRIUMF’s strategy involves using both existing and new proton beamlines from its 520 MeV cyclotron, along with a newly built 30 MeV electron linac in the upcoming Advanced Rare IsotopE Laboratory (ARIEL) facility, to irradiate thorium and uranium targets to produce a variety of radiometals. These include 225Ra and 224Ra, which are parent isotopes for the daughter products 225Ac, 212,213Bi and 212Pb. Because these targets can be positioned downstream from the science targets, the symbiotic production of these radiometals is limited only by the beam intensity.

Image credit: Alfred Morgenstern.
Under the envisioned operating conditions of the new proton beamline, FLUKA simulations of the ARIEL proton target station predict yields of several-hundred millicuries of 225Ac per irradiation and significant quantities of other isotopes. While only very small quantities of 225Ac are required for radionuclide therapy, larger quantities are required to produce enough 213Bi in those treatments where it’s preferred. A larger demand for 213Bi will then drive a similarly increased demand for 225Ac to provide adequate 225Ac/213Bi generators. Thus, TRIUMF’s emerging production capacity would yield sufficient 225Ac to enable the assembly of multiple 225Ac/213Bi generators for therapeutic research studies in patients at multiple centres. Based on typical operating-schedule estimates, this technique could result in the production of several curies of 225Ac per year, compared to the current global output of 1 to 2 curies per year, making the proposed infrastructure a potentially potent source of this valuable isotope. Furthermore, many other medically relevant radioisotopes apart from 225Ac are produced from a thorium or uranium target. The higher current proton beam at ARIEL will enable TRIUMF researchers to explore this exciting medical isotope further.

Image credit: TRIUMF
The ultimate goal of TRIUMF’s TRT programme is to carry out clinical testing and establish the efficacy of TRT agents, enabling a national and possibly international clinical-trial programme for promising therapeutics. TRIUMF research partners will develop new radiopharmaceuticals incorporating therapeutic nuclei into targeting molecules, producing therapeutic conjugates that are used to shepherd their targeted payload to tumours. In addition, research will be carried out to design new molecules that can be used to target different types of tumours.
By leveraging TRIUMF’s existing infrastructure and established research partnerships, the medical community can look forward to production of higher quantities of TRT isotopes. Should the promising results seen to date materialise into a viable treatment option for late stage and/or currently untreatable cancers, the results will bring new hope for a significant number of cancer patients worldwide.