A new hybrid-imaging technique could link particle physics and industry.
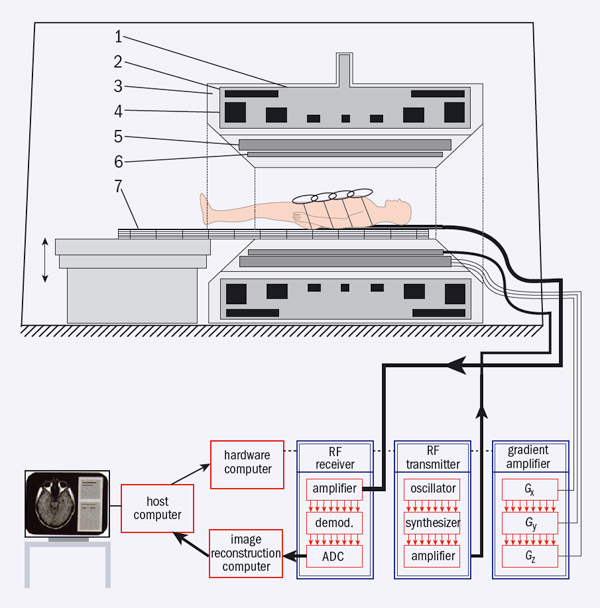
Image credit: Springer Science+ Business Media: Magnetic Resonance Tomography by M F Reiser, W Semmlar and H Hricak (eds) 2008 p77.
Modern medical imaging of the human body often provides not only anatomical detail but also functional information or the biochemical status of a particular region of the body. The first example of the combined use of anatomical and functional imaging, now known as “hybrid imaging”, put positron emission tomography (PET) together with computed tomography (CT). David Townsend, a former CERN scientist working at the University Hospital in Geneva, first thought of incorporating an X-ray-based CT scanner in the same instrument as a PET camera in 1991. The first such instrument was in operation by the end of the 1990s and now all PET cameras that are commercially available from the major international companies are combined PET/CT scanners.
Although PET/CT has proved its value in oncology, CT still has some serious limitations related to soft-tissue contrast, which often needs additional injections of contrast agents for the patient. In CT imaging, high levels of radiation exposure are also a concern, particularly in paediatrics, in repeated scanning for therapy monitoring and in other non-oncology pathologies. An alternative approach for hybrid imaging has arisen recently with the emergence of systems that combine PET with magnetic-resonance imaging (MRI). The advantages of a PET/MRI scanner is evident from the table below, which illustrates the merits of the different medical-imaging techniques.
Unlike CT, MRI provides good contrast in soft tissue. This technique involves aligning the magnetic moments of hydrogen nuclei in a strong magnetic field and then using a temporary RF field to flip the spin of some of them. When these nuclei revert to their former state, they radiate at the same radio frequency. The key to providing an image is to apply an additional magnetic-field gradient so that the resonant frequency varies with position. A typical MRI scanner comprises a strong magnet to produce a static, homogeneous, longitudinal magnetic field (B0), three “gradient” coils that can be switched on and off, an RF transmitter, RF receiver coils and a computer-control and data-acquisition system (figure 1).
Superconducting magnets offer the optimum way to provide the necessary field strength over the volume required in a whole-body scanner and the commercial development of these magnets from the 1970s onwards has led to the wide medical use of MRI. Modern systems have fields of 1.5–3 T, although some with fields up to 7 T already exist. The gradient coils generate magnetic-field gradients in x, y and z directions and are used to encode position, while the RF-transmitter coil is used to excite nuclei by dragging longitudinal magnetization from the B0 direction to a desired predefined angle. Several RF-receiver coils are used – one is integrated into the MRI scanner and several “surface” coils can be placed closer to the patient to improve signal-to-noise ratios. These coils receive RF pulses transmitted from nuclei when they lose their excitation and their re-alignment with B0. Recently, the RF receiver–transmitter system has evolved to accommodate two parallel transmitters that improve the spatial homogeneity of acquired signals – this is important in 3 T systems for whole-body imaging.
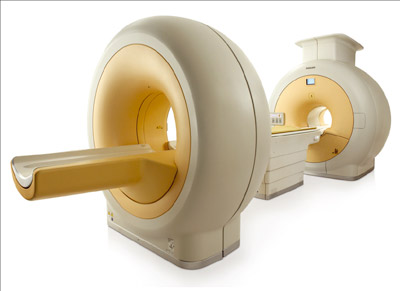
Image credit: Philips Healthcare.
A PET camera, by contrast, is used to detect and measure the distribution in the body of radioisotopes decaying via positron emission. For every positron annihilation event in tissue, an almost coplanar pair of 511 keV gamma-ray photons is produced. A PET detector consists of a pixelated scintillator ring connected to banks of photomultiplier tubes (PMTs) via optical guides. The PMTs convert the photons of visible light from the scintillators into voltage and the relative output voltages of pairs of PMTs determine the position of the photon pairs at the detector surface. To identify photon pairs, the PMTs operate in coincidence using a timing window of a few nanoseconds. The detected coincident pair defines a line-of-response (LOR), somewhere along which a positron annihilation event happened. Detectors with fast timing-resolution, typically less than 600 ps, can localize the annihilation on the LOR using time-of-flight technology (TOF).
PET meets MRI
PMTs, however, are inherently unable to operate inside a magnetic field. Early attempts to develop smaller animal PET/MRI scanners produced several innovative prototypes using different approaches to overcome the cross-talk between the PET and MRI systems. In 2008, both the Philips and Siemens medical companies developed their first PET/MRI prototypes for humans. The Philips system had two independent scanners and additional shielding to contain the magnetic field from its 3 T magnet, with a coaxial distance between the PET detector and the MRI scanner of 4.2 m (figure 2). Furthermore, each PMT was individually shielded and its photocathode aligned along the flux lines of the magnetic field. Apart from this change the PET detector was the same as the commercially available TOF scanner and this PET/MRI system was capable of acquiring whole-body images in a sequential fashion. The Siemens prototype had a PET scanner integrated into an MRI system with a 3 T scanner. A retractable PET detector used avalanche photodiodes (APDs) which are solid-state photon detectors coupled to lutetium oxyorthosilicate scintillator crystals (LSO). The system was designed to acquire simultaneous PET and MRI pictures of the brain.
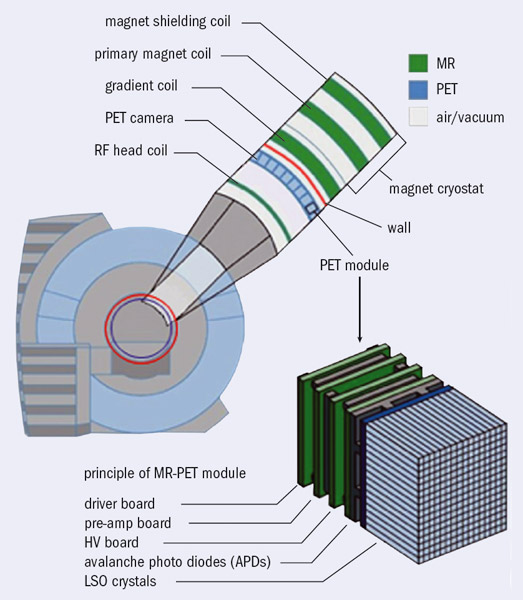
Image credit: Siemens Healthcare. Based on the diagram in Medical Solutions September 2005 p28.
Today, three large-imaging companies have PET/MRI whole-body scanners in their portfolios, although all three are significantly different. In 2010, Siemens announced a whole-body simultaneous PET/MRI scanner based on their original technology and this has already received medical devices registration (CE mark for Europe and 510(k) for the US). This latest model comprises a 70 cm diameter 3 T magnet with an integrated PET detector ring 60 cm in diameter. The PET detector consists of pixelated scintillators coupled via optical guides to an array of APDs (figure 3). APDs are insensitive to magnetic fields and have high gain (102–103) and timing resolution of the order of 1 ns (Lewellen 2008). The APD arrays are connected to front-end electronics for pre-amplification and digitization and have a cooling circuit to maintain constant temperature because their gain is temperature sensitive. Meanwhile, Philips has commercialized its sequential whole-body TOF-PET/MRI system (CE mark already received and 510(k) approval pending). A third company, General Electric, is proposing an arrangement where an MRI scanner is placed in a room adjacent to a PET/CT scanner and the patient is transferred from one system to the other using a shuttle couch arrangement.

Some concerns remain about the integration of MRI and PET. Photons travelling through a patient’s body are absorbed or attenuated and are not registered. In PET/CT systems, this is compensated for by using a low-dose CT scan, which provides an accurate attenuation map of the object being imaged; this CT attenuation map can then be used for attenuation correction of the PET image. This is not possible in PET/MRI systems and various methods for estimating attenuation coefficients are still under development. Another problem is cross-talk between PET and MRI because RF pulses from the MRI may cause the PET electronics to lose counts during transmission of the RF pulses. Nevertheless, the latest commercial systems seem to have overcome most of the problems and fine-tuning of the designs continues. Industry and the medical-imaging community are now actively collaborating to use and improve this new medical technology, as well as to demonstrate a true clinical utility for PET/MRI scanners. This has already resulted in a multitude of scientific publications on these topics in both journals and conferences on PET and MRI.
There is a remarkable similarity in design between these integrated PET/MRI clinical scanners and the large, general-purpose detector systems developed in particle physics. For example, the CMS experiment at the LHC in CERN has a central detector of 4 m × 15 m within an axial magnetic field of 4 T (figure 4). This can be compared with a commercial whole-body PET/MRI scanner, with a field of view of 0.6 m × 0.26 m and magnetic fields of up to 3 T. It is therefore reasonable to expect that the latest technologies now being used in particle physics detectors – e.g. silicon photomultipliers – will soon be incorporated by industry into newer and more sensitive combined PET/MRI scanners, with timing-resolution capabilities superior to even those of today’s state-of-the-art PET/CT scanners that are based on PMTs.