Knowledge gained in developing particle detectors for the LHC has been used to create a dedicated PET device for breast scans.
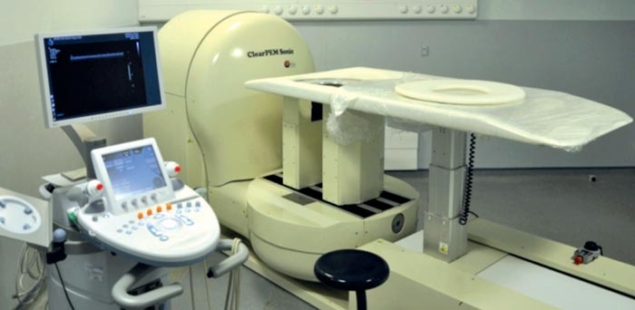
Image credit: B Frisch 2011.
Breast cancer is the most frequent type of cancer among women and accounts for up to 23% of all cancer cases in female patients. The chance of a full recovery is high if the cancer is detected while it is still sufficiently small and has not had time to spread to other parts of the body. Routine breast-cancer screening is therefore part of health-care policies in many advanced countries. Conventional imaging techniques, such as X-ray, ultrasound or magnetic resonance imaging (MRI), rely on anatomical differences between healthy and cancerous tissue. For most patients, the information provided by these different modalities is sufficient to establish a clear diagnosis. For some patients, however, the examination will be inconclusive – for example, because their breast tissue is too dense to allow for a clear image – so these people will require further exams. Others may be diagnosed with a suspicious lesion that requires a biopsy for confirmation. Yet, once this biopsy is over, it might turn out to have been a false alarm.
Patients in this latter category can benefit from nuclear medicine. Positron-emission tomography (PET), for example, offers an entirely different approach to medical imaging by focusing on differences in the body’s metabolism. PET uses molecules involved in metabolic processes, which are labelled by a positron-emitting radioisotope. The molecule, once injected, is taken up in different proportions by healthy and cancerous cells. The emitted positrons annihilate with electrons in the surrounding atoms and produce a back-to-back pair of γ rays of 511 keV. The γ radiation is detected to reveal the distribution of the isotope in the patient’s body. However, whole-body PET suffers from a low spatial resolution of 5–10 mm for most machines, which is too coarse to allow for a precise breast examination. Several research groups are therefore aiming to produce dedicated systems, known as positron-emission mammographs (PEM), that have a resolution better than 2 mm.
One of these groups is the Crystal Clear collaboration (CCC), which is developing a system called ClearPEM. Founded in 1990 as project RD-18 within CERN’s Detector Research and Development Committee’s programme, the CCC aimed at R&D on fast, radiation-hard scintillating crystals for calorimetry at the LHC (Lecoq 1991). In this context, the collaboration contributed to the successful development of the lead tungstate (PbWO4) crystals now used in the electromagnetic calorimeters in the CMS and ALICE experiments at the LHC (Breskin and Voss 2009).
The CCC has transferred its knowledge to medical applications
Building on this experience, the CCC has transferred its knowledge to medical applications – initially through the development of a preclinical scanner for small animals, the ClearPET (Auffray et al. 2004. Indeed, the technical requirements for PET are close to those of applications in high-energy physics. Both require fast scintillators with high light-output and good energy resolution. They need compact and efficient photodetectors that are read by highly integrated, low-noise electronics that can treat the signals from thousands of channels. The CCC also has expertise in co-ordinating an international collaboration to develop leading-edge scientific devices.
Recently, the collaboration has used the experience gained with ClearPET to develop a dedicated PET system for human medicine – the ClearPEM, shown in figure 1 (Lecoq and Varela 2002). The breast was chosen as a target organ because of the benefits related to precise diagnosis of breast cancer. With the ClearPEM, the patient lies in a prone position on a bed designed such that the breast hangs through a hole. A robot moves the bed into position over two parallel detector-plates that rotate around the breast to acquire a full 3D image. In addition, ClearPEM also performs examinations of the armpit – the axilla – by rotating its detector arm by 90 degrees, thereby shifting the plates to be on each side of it.
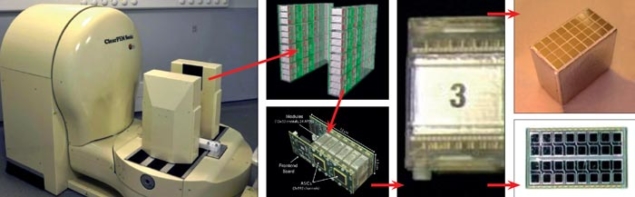
Each detector plate contains 96 detector matrices, where one matrix consists of an 8 × 4 array of cerium-doped lutetium-yttrium silicate (LYSO:Ce) crystals, each 2 × 2 × 20 mm3 in size. As figure 2 shows, each crystal matrix is coupled to two 8 × 4 arrays of Hamamatsu S8550 avalanche photodiode (APD) arrays, such that every 2 × 2 mm2 read-out face is coupled to a dedicated APD. This configuration allows the depth of interaction (DOI) in the crystals to be measured and reduces the parallax error of the lines of response, contributing to better spatial resolution in the reconstructed image. The DOI can be measured with an uncertainty of around 2 mm on the exact position of the γ interaction in the crystal. Each signal channel is coupled to one input of a dedicated 192-channel ASIC, developed by the Portuguese Laboratory for Particle Physics and Instrumentation (LIP). It provides front-end treatment of the signal before handing it over to a 10-bit sampling ADC for digitalization (Varela et al. 2007). The image is reconstructed with a dedicated iterative algorithm.
Two ClearPEM prototypes have been built. The first is currently installed at the Instituto de Ciências Nucleares Aplicadas à Saúde in Coimbra, Portugal. The second, installed at Hôpital Nord in Marseilles, France, is used for ClearPEM-Sonic, a project within the European Centre for Research in Medical Imaging (CERIMED) initiative. While ClearPEM provides high-resolution metabolic information, it lacks anatomical details. ClearPEM-Sonic, however, extends the second prototype with an ultrasound elastography device, which images strain in soft tissue (Frisch 2011). The aim is to provide multimodal information that reveals the exact location of potential lesions in the surrounding anatomy. The availability of elastographic information further improves the specificity of the examination by identifying non-cancerous diseases – such as benign inflammatory diseases of the breast – that exhibit higher uptake of the radioactive tracer, fluorodeoxyglucose (18F), or FDG, used in PET imaging.
The French authority has approved ClearPEM-Sonic for a first clinical trial on 20 patients
Both prototypes have been tested extensively. The electronic noise level is under 2%, with an interchannel noise dispersion of below 8%. The front-end trigger accepts signals at a rate of 2.5 MHz, while the overall acquisition rate reaches 0.8 MHz. The detector has been properly calibrated and gives an energy resolution of 14.6% FWHM for 511 keV photons, which allows for efficient rejection of photons that have lost energy during a scattering process. The coincidence-time resolution of 4.6 ns FWHM reduces the number of random coincidences. The global detection efficiency in the centre of the plates has been determined to be 1.5% at a plate distance of 100 mm. The image resolution measured with a dedicated Jasczcak phantom is 1.3 mm.
The competent French authority has approved ClearPEM-Sonic for a first clinical trial on 20 patients. The goal of this trial is to study the feasibility and safety of PEM examinations. In parallel, the results of ClearPEM are being compared with other modalities, such as classical B-mode ultrasound, X-ray mammography, whole-body combined PET and computerized tomography (PET/CT) imaging and MRI, which all patients participating in this trial will have undergone. The ClearPEM image is acquired immediately after the whole-body PET/CT, which avoids the need for a second injection of FDG for the patient. The histological assessment of the biopsy is used as the gold standard.

The sample case study shown in figure 3 is a patient who was diagnosed with multifocal breast cancer during the initial examination. The whole-body PET/CT reveals a first lesion in the left breast and a second close to the axilla. Before deciding on the best therapy, it was crucial to find out whether the cancer had spread to the whole breast or was still confined to two individual lesions. An extended examination with MRI shows small lesions around the first one. The whole-body PET/CT image, however, does not show any small lesions. The standard procedure is to obtain biopsy samples of the suspicious tissue. However, the availability of a high-resolution PET can give the same information. Indeed, when the patient was imaged with ClearPEM, the lesions visible with MRI were confirmed to be metabolically hyperactive, i.e. potentially cancerous. The biopsy subsequently conducted confirmed this indication. This clinical case study, together with several others, hints at how ClearPEM could improve the diagnostic process.
This project successfully demonstrates the value of fundamental research in high-energy physics in applications to wider society. The knowledge gained by an international collaboration in the development of particle detectors for the LHC has been put to use in the construction of a new medical device – a dedicated breast PET scanner, ClearPEM. It provides excellent image resolution that allows the detection of small lesions. Its high detection efficiency allows a reduction in the total examination time and in the amount of radioactive tracer that has to be injected. Last, first clinical results hint at the medical value of this device.
• The members of the ClearPEM-Sonic collaboration are: CERN; the University of Aix-Marseille; the Vrije Universiteit Brussels; the Portuguese Laboratory for Particle Physics and Instrumentation, Lisbon; the Laboratoire de Mecanique et Acoustique, Marseille; the University Milano-Biccoca; PETsys, Lisbon; SuperSonic Imagine, Aix-en-Provence; AssistancePublique – Hôpitaux de Marseille; and the Institut Paoli Calmettes, Marseille.