A pioneering experiment at CERN with potential for cancer therapy has produced its first results. Exploiting the unique capability of CERN’s Antiproton Decelerator to produce an antiproton beam at the right energy, the Antiproton Cell Experiment (ACE) has shown that antiprotons are four times more effective than protons for cell irradiation.
Cancer therapy is about collateral damage: destroying the tumour while avoiding the healthy tissue around it. Unwanted exposure of healthy tissue could cause side effects and result in a reduced quality of life. It is also believed to increase the chances of secondary cancers developing. In radiation therapy there is an ongoing quest to reduce the radiation level to tissue outside the primary tumour volume.

In hadron therapy, which began in 1946 with Robert Wilson’s seminal paper, “Radiological Use of Fast Protons”, the dose profile of heavy charged particles (hadrons) does not irradiate healthy tissue because most of the energy is deposited at the end of the flight path of the particles – the Bragg peak – with little before and none beyond. However, the question remains of how to maximize the concentration of energy onto the target.
The first speculations that antiprotons could offer a significant gain in targeting tumours through the extra energy released by annihilation date back more than 20 years (Gray and Kalogeropoulos 1984). Now the ACE collaboration has tested this idea by directly comparing the effectiveness of cell irradiation using protons and antiprotons.
To simulate a cross-section of tissue inside a body, the experiment uses tubes filled with live hamster cells suspended in gelatine. These are irradiated with beams of protons or antiprotons at a variety of intensities with about a 2 cm range in water. After irradiation the gelatine is extruded from the tubes and cut into 1 mm slices. These are then dissolved in growth medium and the cells are placed in Petri dishes in an incubator. After a few days the naked eye can see that some of the cells have produced healthy offspring. This gives a measure of the survival of cells along the beam path for the different dose levels. Cell survival is plotted for the entrance and the Bragg-peak regions as a function of particle fluencies, and the ratio of dose for a 20% survival in these two regions is extracted.
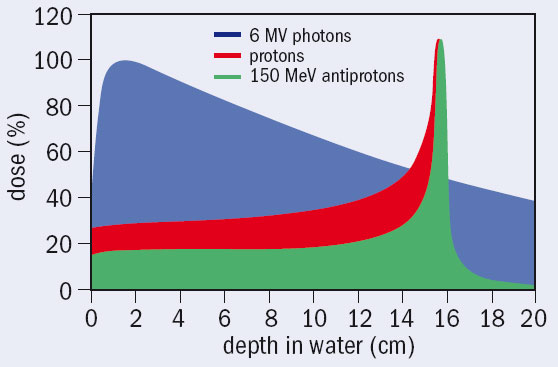
Comparing beams of protons and antiprotons that cause identical damage at the entrance to the target, the results of the experiment show that the damage to cells inflicted at the end of the beam is four times higher for antiprotons (Holzscheiter et al. 2006.) The method directly samples the total effect of the beams on the cells, combining the enhanced energy deposition in the vicinity of the annihilation point and the higher biological effectiveness of this extra energy (delivered by nuclear fragments). The experiment demonstrates a significant reduction of the damage to the healthy cells along the entrance channel of a beam for antiprotons compared with protons.
While antiprotons may seem unlikely candidates for cancer therapy, the initial results from ACE indicate that these antimatter particles could lead to more effective radiation therapy. There is no doubt, however, that the first clinical application is still at least a decade away.
Further reading
L Gray and T E Kalogeropoulos 1984 Radiation Research 97 246.
M H Holzscheiter et al. 2006 Radiother. Oncol. doi:10.1016/j.radonc.2006.09.012.