The Next Ion Medical Machine Study (NIMMS) aims to leverage CERN technologies and expertise in accelerators to design a new generation of light-ion accelerators for medicine.
Twenty years ago, pioneering work at CERN helped propel Europe to the forefront of cancer treatment with hadron beams. The Proton Ion Medical Machine Study (PIMMS), founded in 1996 by a CERN–TERA Foundation-MedAustron–Oncology2000 collaboration, paved the way to the construction of two hadron-therapy centres: CNAO in Pavia (Italy) and MedAustron in Wiener Neustadt (Austria). A parallel pioneering development at GSI produced two similar centres in Germany (HIT in Heidelberg and MIT in Marburg). Since the commissioning of the first facility in 2009, the four European hadron-therapy centres have treated more than 10,000 patients with protons or carbon ions. The improved health and life expectancy of these individuals is the best reward to the vision of all those at CERN and GSI who laid the foundations for this new type of cancer treatment.
Almost four million new cancer cases are diagnosed per year in Europe, around half of which can be effectively treated with X-rays at relatively low cost. Where hadrons are advantageous is in the treatment of deep tumours close to critical organs or of paediatric tumours. For these cancers, the “Bragg peak” energy-deposition characteristic of charged particles reduces the radiation dose to organs surrounding the tumour, increasing survival rates and reducing negative side effects and the risk of recurrency. With respect to protons, carbon ions have the additional advantages of hitting the target more precisely with higher biological effect, and of being effective against radioresistant hypoxic tumours, which constitute between 1 and 3% of all radiation-therapy cases. Present facilities treat only a small fraction of all patients who could take advantage of hadron therapy, however. The diffusion of this relatively novel cancer treatment is primarily limited by its cost, and by the need for more pre-clinical and clinical research to fully exploit its potential.
Given these limitations, how can the scientific community contribute to extending the benefits of hadron therapy to a larger number of cancer patients? To review this and similar questions, CERN has recently given a new boost to its medical accelerator activities, after a long interruption corresponding to the time when CERN resources where directed mainly towards LHC construction. The framework for this renewed effort was provided by the CERN Council in 2017 when it approved a strategy concerning knowledge-transfer for the benefit of medical applications. This strategy specifically encouraged new initiatives to leverage existing and upcoming CERN technologies and expertise in accelerator technologies towards the design of a new generation of light-ion accelerators for medicine.

The hadron-therapy landscape in 2020 is very different from what it was 20 years ago. The principal reason is that industry has entered the field and developed a new generation of compact cyclotrons for proton therapy. Beyond the four hadron (proton and ion) centres there are now 23 industry-built facilities in Europe providing only proton therapy to about 4000 patients per year. Thanks to this new set of facilities, proton therapy is now highly developed and is progressively extending its reach in competition with more conventional X-ray radiation therapy.
Despite its many advantages over X-rays and protons, therapy with ions (mainly carbon, but other ions like helium or oxygen are under study) is still administered in Europe only by the four large hadron-therapy facilities. In comparison, eight ion-therapy accelerators are in operation in Asia, most of them in Japan, and four others are under construction. The development of new specific instruments for cancer therapy with ions is an ideal application for CERN technologies, in line with CERN’s role of promoting the adoption of cutting-edge technologies that might result in innovative products and open new markets.
Next-generation accelerators
To propel the use of cancer therapy with ions we need a next-generation accelerator, capable of bringing beams of carbon ions to the 430 MeV/u energy required to cover the full body, with smaller dimensions and cost compared to the PIMMS-type machines. A new accelerator design with improved intensity and operational flexibility would also enable a wide research programme to optimise ion species and treatment modalities, in line with what was foreseen by the cancelled BioLEIR programme at CERN. This would allow the exploration of innovative paths to the treatment of cancer such as ultra-short FLASH therapy or the promising combination of ion therapy with immunotherapy, which is expected to trigger an immune response against diffused cancers and metastasis. Moreover, a more compact accelerator could be installed in, or very close to, existing hospitals to fully integrate ion therapy in cancer-treatment protocols while minimising the need to transport patients over long distances.
The development of new specific instruments for cancer therapy with ions is an ideal application for CERN technologies
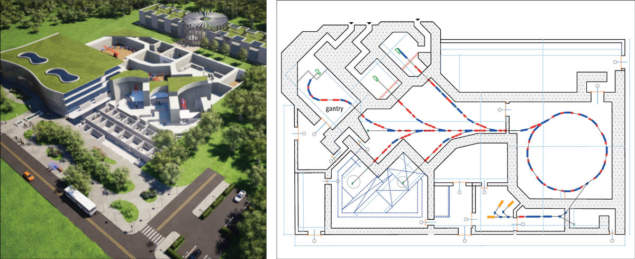
These considerations are the foundation for the Next Ion Medical Machine Study (NIMMS), a new CERN initiative that aims to develop specific accelerator technologies for the next generation of ion-therapy facilities and help catalyse a new European collective action for therapy with ion beams. The NIMMS activities were launched in 2019, following a workshop at ESI Archamps in 2018 where the medical and accelerator communities agreed on basic specifications for a new-generation machine. In addition to smaller dimensions and cost, these include a higher beam current for faster treatment, operation with multiple ions, and irradiation from different angles using a gantry system.
In addressing the challenges of new designs with reduced dimensions, CERN is building on the development work promoted in the last decade by the TERA Foundation. Reducing the accelerator dimensions from the conventional synchrotrons used so far can take different directions, out of which two are particularly promising. The first is the classic approach of using superconductivity to increase the magnetic field and decrease the radius of the synchrotron, and the second consists of replacing the synchrotron with a high-gradient linear accelerator with a new design – in line with the proton therapy linac being developed by ADAM, a spin-off company of CERN and TERA now part of the AVO group. The goal in both designs is to reduce the surface occupied by the accelerator by more than a factor of two, from about 1200 to 500 m2. With these considerations in mind, the NIMMS study has been structured in four work packages.
The main avenue to reduced dimensions is superconductivity, and the goal of the first work package is to develop new superconducting magnet designs for pulsed operation, with large apertures and curvatures – suitable for an ideal “square” synchrotron layout with only four 90 degree magnets. Different concepts are being explored, with some attention to the so-called canted cosine-theta design (see “Combined windings”) used for example in orbit correctors for the high-luminosity LHC, of which a team at Lawrence Berkeley National Laboratory has recently developed a curved prototype for medical applications. Other options under study are based on more traditional cosine-theta designs (see “Split yoke”), and on exploiting the potential of modern high-temperature superconductors.
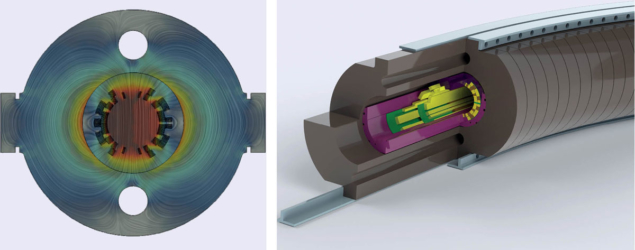
The second work package covers the design of a compact linear accelerator optimised for installation in hospitals. Operating at 3 GHz with high field gradients, this linac design profits from the expertise gained with accelerating structures developed for the proposed Compact Linear Collider (CLIC), and uses as an injector a novel source for fully-stripped carbon based on the REX-ISOLDE design. The source is followed by a 750 MHz radio-frequency quadrupole using the design recently developed at CERN for medical and industrial applications.
The third NIMMS work package focuses on compact superconducting designs for the gantry, the large element required to precisely deliver ion beams to the patient that is critical for the cost and performance of an ion-therapy facility. The problem of integrating a large-acceptance beam optics with a compact superconducting magnetic system within a robust mechanical structure is an ideal challenge for the expertise of the CERN accelerator groups. Two designs are being considered: a lightweight rotational gantry covering only 180 degrees originally proposed by TERA, and the GaToroid toroidal gantry being developed at CERN.
NIMMS will consider new designs for the injector linac, with reduced cost and dimensions
The fourth work package is dedicated to the development of new high-current synchrotron designs, and to their integration in future cancer research and therapy facilities. To reduce treatment time, the goal is to accelerate more than an order of magnitude higher current than in the present European facilities. This requires careful multi-turn injection into the ring and strict control of beam optics, which add to other specific features of the new design, including a fast extraction that will make tests with the new ultra-fast FLASH treatment modality possible. Two synchrotron layouts are being considered, a more conventional one with room-temperature magnets (see “Ions for therapy”), and a very compact superconducting one of only 27 m circumference. The latter, equipped with a gantry of new design, would allow a single-room carbon-therapy facility to be realised in an area of about 1000 m2. Additionally, NIMMS will consider new designs for the injector linac, with reduced cost and dimensions and including the option of being used for production of medical radioisotopes – for imaging and therapy – during the otherwise idle time between two synchrotron injections.
Ambitious work plan
This ambitious work plan exceeds the resources that CERN can allocate to this study, and its development requires collaborations at different levels. The first enthusiastic partner is the new SEEIIST (South East European International Institute for Sustainable Technologies) organisation, which aims at building a pan-European facility for cancer research and therapy with ions (see “Ions for therapy”). SEEIIST is already joining forces with NIMMS by supporting staff working at CERN on synchrotron and gantry design. The second partnership is with the ion therapy centres CNAO and MedAustron, which are evaluating the proposed superconducting gantry design in view of extending the treatment capabilities of their facilities. A third critical partner is CIEMAT, which will build the high-frequency linac pre-injector and validate it with beam. Other partners participating in the study at different levels are GSI, PSI, HIT, INFN, Melbourne University, Imperial College, and of course TERA which remains one of the driving forces behind medical-accelerator developments. This wide collaboration has been successful in attracting additional support from the European Commission via two recently approved projects beginning in 2021. The multidisciplinary HITRIplus project on ion therapy includes work packages dedicated to accelerator, gantry and superconducting magnet design, while the IFAST project for cutting-edge accelerator R&D contains an ambitious programme focusing on the optimisation and prototyping of superconducting magnets for ion therapy with industry.
Every technology starts from a dream, and particle accelerators are there to fulfil one of the oldest: looking inside the human body and curing it without bloodshed. It is up to us to further develop the tools to realise this dream.