PROSCAN, the proton-therapy facility at PSI in Switzerland is about to resume patient treatment after commissioning a new dedicated superconducting proton accelerator, COMET. This will take the project into a new technological and clinical phase, as Peter-Raymond Kettle explains.
In Europe, one in three people is expected to confront some form of cancer during his or her lifetime. In Switzerland alone, this amounts to about 28,000 patients a year – 70% of whom undergo radiotherapy, now the second most successful form of treatment after surgery. While the majority of tumours are treated with photons, the use of proton beams, first proposed 60 years ago, is becoming increasingly important for deep-seated tumours, as the technique moves from accelerator centres to dedicated clinical facilities. The Paul Scherrer Institute (PSI) is one of Europe’s leading centres for proton-therapy research and has recently begun work with a new superconducting proton cyclotron, COMET, and beamline to serve its proton-therapy project, PROSCAN.
PROSCAN grew from PSI’s decision in 2000 to expand its radiotherapy activities by developing a new compact gantry system to enable the laboratory’s successful spot-scanning technology to be used in a hospital environment. The project also aims at optimizing treatment methods and expanding the spectrum of treatable forms of cancers, as well as transferring the technology and know-how to industry and other radiotherapy centres, including the education and training of specialist personnel.
PSI’s pioneering development of the spot-scanning technique for deep-seated tumours dates back to its former times as the Swiss Institute for Nuclear Research (SIN), when the laboratory established a therapy programme using pions. This used a dynamic beam-delivery system of 60 converging pion beams where the patient’s target area could be moved 3-dimensionally within a body (bolus) of water. Some 500 patients were treated with this system between 1981 and 1992. Protons for both the pion work and the subsequent proton-therapy programme at the Gantry 1 system described below were produced by the main 590 MeV Ring Cyclotron and a beam-splitter system, which at the same time served the main users in particle physics and materials science. In 1984, in collaboration with the Lausanne University Eye Clinic, the very successful OPTIS Proton Therapy Programme began. A first in Europe, this facility has treated more than 4400 patients with differing forms of eye tumours, using a 70 MeV horizontal proton beam from PSI’s Injector 1 cyclotron. With a success rate of better than 98%, it continues to treat the highest number of eye patients worldwide each year, and has led in turn to the establishment and operation of six new European facilities in England, France, Germany, Italy and Sweden.
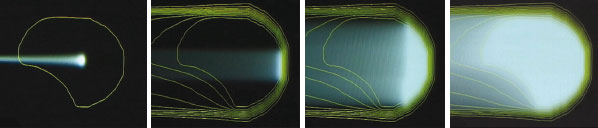
Modern ways to achieve a conformal dose distribution tailored 3-dimensionally to a target tumour volume, while sparing healthy tissue, use active scanning rather than conventional passive scattering (Goitein et al. 2002). The latter, which has fewer degrees of freedom, uses a set of scattering foils to produce a laterally spread-out proton beam of constant range. A set of individually manufactured collimators and compensators then contours the beam to match each target volume. The range of the protons is subsequently modulated by, for example, a rotating range-shifter wheel, which alters the proton depth profile.
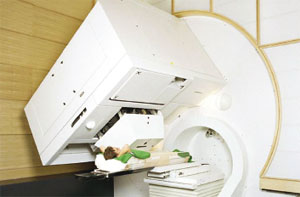
Figure 1 illustrates the principle of active scanning. Here the dose delivery to the patient is achieved through the sequential superposition of single pencil beams of protons, each of which produces a hot spot at the Bragg peak, where the protons deposit most of their energy. The hot spot is about 1 cm3 for a Gaussian beam profile of 7–8 mm FWHM. Lateral scanning is possible either using sweeper magnets or by moving the patient table, or by a combination of both. Depth modulation, on the other hand, is achieved either by a fast active degrader or by changing the beam energy. Combining these options with both a beam-delivery system that can rotate and an eccentrically mounted counter-rotating patient table yields the very compact (4 m diameter) PSI Gantry 1 system (figure 2).
This system allows a dose application of almost 10,000 spots/litre to be applied in a few minutes with an individual spot-dose precision of 1%. It is the only facility in the world to use a dynamic beam-delivery technique based on active spot scanning with protons. A similar scanning system using a horizontal beamline has been developed for carbon ions at Germany’s national laboratory for heavy-ion physics, GSI in Darmstadt.

Gantry 1 was designed to treat deep-seated tumours and since it started up in 1996 has handled around 260 patients, some 69 of whom were treated with a new therapy plan using intensity-modulated proton therapy (IMPT), which further reduces the dose to healthy tissue. One of the main disadvantages of the present spot-scanning technique as applied in Gantry 1 is the modulation speed, which is currently too slow to apply IMPT to moving target volumes. This will soon be addressed with the new features being incorporated into Gantry 2, within the PROSCAN project.
The PROSCAN project
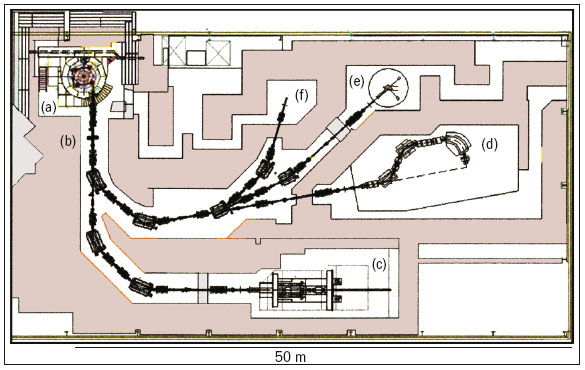
When PROSCAN is complete it will consist of six main features (figure 3), with the newly commissioned 250 MeV superconducting cyclotron, COMET, as the heart of the facility (figure 4). ACCEL Instruments, Germany, built this machine in close co-operation with accelerator specialists at PSI, and based it on a design by Henry Blosser of Michigan State University. It will allow year-round therapy operation. The decision in favour of a cyclotron was based on the benefits of the 100% duty cycle and the need to control the beam intensity precisely and dynamically prior to acceleration. The restriction of the fixed energy from a cyclotron in turn requires a degrader system that acts rapidly, allowing fast, small energy steps to be implemented. This, together with a beam-diagnostics system after the degrader, means that the rapid modulation of both energy and intensity can be fully exploited, allowing the possibility of fast volumetric rescanning and the study of IMPT for moving tumours. The new machine also achieved the stringent requirement of an 80% extraction efficiency and an availability of 98%.

Gantry 1 will remain unchanged and will continue as the workhorse for treating patients in PROSCAN. The development work will concentrate on implementing IMPT methods into clinical practice, including treatment planning, dosimetry and quality-assurance. The present OPTIS eye-treatment facility at the Injector 1 cyclotron will continue its successful operation until mid-2007 when it will be transferred to the new 70 MeV area at PROSCAN. This will involve a complete re-design of the control system and treatment procedures. The second of the two horizontal beamlines will do biological and dosimetry experiments.
Finally, to meet the challenge to proton therapy of producing a beam-scanning method that can overcome the sensitivity to organ motion, a new compact gantry system, Gantry 2, is being built to be implemented in 2007, with the first treatments of patients expected in 2008 (figure 5). This will allow faster beam scanning by 2D magnetic deflection, to achieve multiple target rescannings of the same volume within a single sitting (Pedroni et al. 2004).
Once the PROSCAN facility is fully operational it is expected that the number of patient treatments for deep-seated tumours will increase by a factor of 3–4 (150–250 patients a year) with a further 200–300 patients a year benefiting from the OPTIS eye- treatment station. It has taken some 50 years from the basic idea for protons to come of age as a clinical tool, enabling more than 40,000 patients so far to benefit from this therapy developed in a multi-disciplinary fashion. Although the future is clearly aimed at providing dedicated commercial facilities for hospitals and clinics, the present role played by accelerator laboratories such as PSI, in developing new methods and the technology to implement them, is an essential ingredient in achieving this goal.
Further reading
For further information about proton therapy at PSI see http://p-therapie.web.psi.ch/e/.
M Goitein et al. 2002 Physics Today 55 (9) 45.
E Pedroni et al. 2004 Z. Med. Phys. 14 25.