Sixty years ago accelerator pioneer Robert Wilson published the paper in which he proposed using protons for cancer therapy. Ugo Amaldi and Gerhard Kraft describe how the field has since advanced, as an increasing number of accelerators in dedicated clinical centres come online to provide therapy with protons and carbon ions.
In 1945 William Hansen at Stanford University built a disk-loaded linear accelerator that produced 4.5 MeV electrons and was less than a metre long. This first electron linac ran at the previously unimaginable frequency of 3 GHz and was so short because it used pulsed high-power klystrons invented by the Varian brothers and developed during the Second World War. Hansen had aimed to advance research in nuclear physics, but his invention was to have an enormous impact on medicine. By the 1970s the company Varian led the market in producing what is now “conventional” radiotherapy systems based on the same type of linac running at the same frequency.
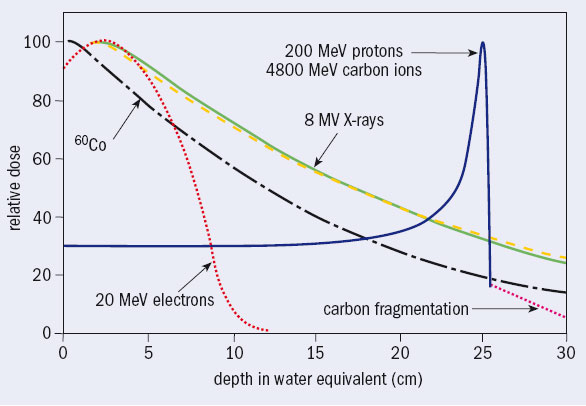
In developed countries every year some 40,000 per 10 million inhabitants are diagnosed as having cancer, around half of whom are treated with high-energy photons produced by electron linacs. There are almost 10,000 electron linacs worldwide, which run more than one shift a day. They irradiate around 4 million patients a year, each in about 30 sessions over 5–6 weeks. The photon beams have energies of a few million electron volts, but are still called X-rays by medical doctors. They have replaced low-energy X-rays and the gamma radiation from radioactive cobalt because they deposit the dose (the energy per unit mass) at greater depth (see figure 1).
In the same year of Hansen’s invention, and not far away, Robert R Wilson – a Harvard associate professor who was working on cyclotrons with his old teacher Ernest Lawrence at the Radiation Laboratory in Berkeley – was computing the shielding thickness for a 150 MeV cyclotron to be constructed and installed at Harvard. Fifty years later, opening the Advances in Hadron Therapy conference, held at CERN in 1996, Wilson said, “I found that a few inches of lead would fix everything. But I did not stop. Why? Fifty years later I do not know why I did not stop. I suppose the first reason was just plain simple curiosity. So I went on and I jumped into the most obvious thing I could do next: because one could hurt people with protons, one could probably help them too. So I tried to work out every detail and I was surprised to see that the Bragg curve came up and came down very sharply,” (Wilson 1997). The narrow Bragg peak at the end of the range (figure 1) prompted him to publish in the journal Radiology a now-famous paper suggesting the use of protons (and carbon ions) to irradiate tumours while sparing – much better than with X-rays – the healthy tissue traversed, contiguous and located more deeply (Wilson 1946).

However, the resonance within the medical community was almost zero and it was a decade before Berkeley and Harvard treated patients with proton beams from accelerators originally designed for nuclear-physics experiments. It wasn’t until the beginning of the 1990s that radiation oncologists started to recognize this new therapeutic method, because the apparatus was huge by medical standards and the irradiations were done in nuclear-physics laboratories with horizontal particle beams and simple beam-shaping methods. By 1993 about 10 000 patients worldwide had been treated with protons, and by the end of 2006 this has reached 50,000. Today five companies supply turnkey proton-therapy centres.
It is no surprise that from 1961 interesting clinical results for proton therapy were obtained at Harvard where radiotherapists at Massachusetts General Hospital and physicists from Harvard have successfully treated many thousands of head and neck tumours. Eventually in 1993 at the Loma Linda University Medical Center in California, the first proton synchrotron dedicated to proton therapy began irradiating patients in three treatment rooms featuring magnet beamlines on 10 m high gantries, which rotate around the patient. Again, it is no surprise that the Loma Linda synchrotron was built at Fermilab, the particle-physics laboratory that Wilson created and then directed until 1987.
Carbon ions join the fight
Heavier ions than protons, such as helium and later argon, first came into use at Berkeley in 1957 and 1975, respectively. At the old 184 inch cyclotron 2800 patients received brain treatment with helium beams: the lateral spread and range straggling are smaller and this leads to much better dose gradients than protons. At the Bevalac, argon beams were tried to increase the effectiveness against hypoxic and otherwise radio-resistant tumours, i.e. tumours that need deposited doses 2–3 times higher if they are to be controlled with either photons or protons. But problems arose owing to non-tolerable side effects in the normal tissues. After a few irradiations, the Bevalac used lighter ions, first silicon ions and then neon, for 433 patients before it shut down in 1993.
The transition from protons to heavier ions adds another order of magnitude to the complexity of patient irradiation. In the beginning at Berkeley the increase in the relative biological effectiveness (RBE) for ions with respect to photons was believed to be related to the physical parameters of the beam, being the same for different tissues. Since 1980 a large programme of systematic studies of RBE has been carried out at various accelerators, such as Unilac (Darmstadt), Ganil (Caen), Bevalac (Berkeley), the Tandem Van de Graff (Heidelberg) and, later, SIS (Darmstadt). This research studied the effects on very different biological objects, from sub-cellular systems, such as DNA and chromosomes, to biological systems that are resistant to extreme environmental conditions and are used in space research.
The experiments used more than 100,000 biological samples and ion beams from very light to very heavy elements. The research identified the systematic dependence of RBE on physical and biological parameters – mainly the capacity of cells to repair DNA damage – as the most important factor, which was then theoretically modelled for use in treatment planning. In particular, the work showed that for beams of carbon ions the section of the particle track with increased RBE coincides with the few centimetres up to the Bragg peak, while for lighter ions it is concentrated in the last few millimetres. For heavier ions, such as the argon, silicon, and neon ions used previously at Berkeley, it causes significant damage in the normal tissues before the tumour.
For these reasons, in 1994 the synchrotron facility led by Yasou Hirao at the Heavy Ion Medical Accelerator in Chiba (HIMAC), of the National Institute for Radiation Sciences in Japan, treated the first patient with carbon ions, although the accelerator complex was originally designed for ions up to argon.
While an energy of 200 MeV is needed to reach deep-seated tumours (about 25 cm of water equivalent) for protons, 4800 MeV is needed for carbon ions, 24 times higher. Protons beams are obtained either from cyclotrons (normal or superconducting) or from synchrotrons with a diameter of 6–8 m. Currently only synchrotrons are used to produce carbon ions up to 400–430 MeV/u. Their magnetic rigidity is about three times larger than for 200 MeV protons, so synchrotrons of 18–25 m diameter are needed.
Since the end of the 1990s, newly built proton-therapy centres feature isocentric gantries to improve treatment conformity. These avoid high doses to healthy tissue by rotating the beam around the patient as in X-ray treatments. These complex hi-tech systems could not be designed and run effectively and continuously – as is necessary in a hospital environment – were it not for decades of colliding particles and understanding the subatomic world.
Until 1997 relatively simple passive spreading systems were used to produce a spread-out Bragg peak in all hadron-therapy centres. A first scatterer widens the pencil beam while the energy is adapted to the further side of the tumour by appropriate absorbers. More recently, GSI and PSI have developed novel active spreading systems (Haberer et al. 1993 and Pedroni et al. 1995, respectively), which magnetically guide the charged hadrons over the treatment area and modulate the intensity (Intensity Modulated Particle Therapy, or IMPT). All future centres will feature such systems. In particular the ion-therapy centres currently being built at Heidelberg and Pavia have been equipped with the first active beam-delivery system for carbon ions, which restricts the physically and biologically effective end of the track to the target volume (Rossi 2006).
Treating patients
By the beginning of 2006, around 45,000 patients had been treated with proton beams in 12 subatomic physics laboratories and in more than 10 hospital-based proton-therapy centres. (The Particle Therapy Co-ordination Group updates the number of patients treated at see http://ptcog.web.psi.ch Another 10 centres are running or are being built. This shows that proton therapy is booming. At the same time around 2200 patients have been treated with carbon ions at HIMAC, and about 300 at the pilot project proposed by Gerhard Kraft and built at GSI in Darmstadt.
In a conventional treatment with photons of a few million electron volts, a total dose of 60–70 Gy (1 Gy = 1 J/kg) is deposited in a tumour in typically 30 fractions over six weeks. This “fractionation” gives time for re-oxygenation of hypoxic – and therefore radio resistant – tumour cells and allows them to change from radio-resistant stages in the cell cycle to more sensitive stages. In addition, unavoidably irradiated healthy cells have a chance to repair themselves. A proton treatment typically needs the same number of fractions, but allows higher doses to the tumour and thus larger control rates. A larger dose is beneficial because even a 10% increase in the deposited dose generally increases the probability of local control of the tumour by 15–20%.
With carbon ions, the clinical results from Japan and Germany on head, lung, liver and prostate tumours confirm the radiobiological predictions that they have a larger RBE than protons, because their ionization is 24 times higher, which produces multiple double-strand breaks of the DNA of the traversed cell. This damage cannot be repaired, so ion beams are most suited for slow-growing tumours, which are precisely those tumours that are resistant to photons and protons. It is important to note that, since there is only little repair to damage by carbon ions, the fractionation of the dose is not needed as far as tumour inactivation is concerned, but for the normal tissue in the entrance channel fractionation helps to repair the less severe damage. In principle a patient can be treated in 5–10 sessions, reducing both psychological and financial cost. A proton treatment costs 2–3 times more than a conventional treatment, averaging in the West around €6000, but the economy of carbon treatment is different because the shortening of the treatment allows for effective use of the infrastructures. If confirmed by the ongoing clinical trials, this will reduce the cost of treatment and may become one of the main reasons behind any rapid expansion of light-ion therapy in the future. In addition, having little or no side effects reinforces the necessity of active beam-delivery systems for carbon ions, to tailor the dose to the tumour.
In summary, research indicates that carbon-ion beams should be used in the treatment of deep-seated tumours, which are radio resistant both to high-energy photons and to protons. These tumours are thus the targets of choice in a carbon-ion facility, while proton therapy is well adapted to the cases in which a tumour is close to critical organs that cannot be irradiated (Amaldi and Kraft 2005). Groups of radiotherapists in Austria, France, Germany and Italy have applied specific criteria for each tumour site to the national data and made detailed analyses of the number of potential patients (Carbon-ion therapy 2004). The results of these different approaches are consistent. They show that about 1% of the patients treated today with X-rays should be irradiated with protons as the outcomes are definitely better than conventional therapy. In addition, about 12% of X-ray patients would benefit from proton treatment but further clinical trials are needed to quantify the clinical advantages site by site. Lastly, about 3% of X-ray patients would benefit from carbon-ion therapy, but more clinical trials and dose-escalation studies are needed.
Overall, 15% of the approximately 20,000 patients per 10 million inhabitants treated with conventional radiation would receive better treatment with hadron beams. Irradiating these patients would require 3–4 proton treatment rooms (i.e. a centre treating about 1500 patients a year) per 5 million people and a carbon-ion centre per 35 million people. A balanced national programme can therefore make good use of dual centres that accelerate carbon ions and protons and feature fixed ion beams (horizontal, vertical and inclined) and rotating gantries for protons.
Hadron therapy in Europe
In the past five years Europe has made important steps in developing and building hospital-based dual centres for carbon ions and protons. Based on the success of GSI’s pilot project, the Heidelberg Ion Therapy Centre (HIT), designed by GSI, was approved in 2001 and civil engineering work began in November 2003. This centre features two horizontal beams and the first carbon-ion rotating gantry, which is 25 m long and weighs 600 tonnes. The first treatment will be at the end of 2007.
At the end of 1995 Ugo Amaldi, with Meinhard Regler of the Med-Austron project, attracted CERN management’s attention to the design of an optimized synchrotron for light-ion therapy. This was the starting point of a five-year Proton and Ion Medical Machine Study (PIMMS) (Badano et al. 1999 and 2000). As a development of this initiative, in 2002 the Italian health minister financed a second European centre, based on the PIMMS design modified by the TERA Foundation (Fondazione per Adroterapia Oncologica). This is now being built in Pave by the Centro Nazionale di Adroterapia Oncologica Foundation (CNAO) with strong support from INFN (figure 3). It will be ready by the end of 2007.
At the end of 2004 the Austrian authorities approved the Med-Austron project, granting a substantial part of the required funding for the construction of a dual centre in Wiener Neustadt. The tendering procedure to acquire a turnkey carbon-ion facility is now almost complete. Similarly, in May 2005 the French government approved the ETOILE project to be built in Lyon.
In 2002 the initiatives at Heidelberg, Lyon, Pave, Stockholm (where the Karolinska Institute has proposed a similar facility) and Wiener Neustadt all teamed up with the European Society for Radiotherapy, CERN and GSI to form the European Network for Light Ion Therapy, which the European Union financed for three years. The work by this network, and the existence of its potential successor, guarantees that carbon-ion therapy in Europe is on the right track and that the foreseen facilities will be run for the benefit of all European patients. During 2006 a larger group of institutes and hospitals from 15 countries has come together to prepare a new proposal for the EU Framework Programme FP7 under the name ENLIGHT++ (CERN Courier June 2006 p27).
In addition, in January 2006 contracts for a privately financed carbon/proton centre were signed by Rhön–Klinikum–AG, which owns more than 40 German hospitals, including the Giessen-Marburg University clinics, and Siemens Particle Therapy. When it starts up in 2010, the new heavy-ion therapy facility in Marburg (figure 4) will show that hadron therapy with ion and proton beams has left research and arrived in the clinical environment.
Future developments
The unique physical and biological properties of hadron beams are better for patients than the most recent photon image guided radiotherapy (IGRT) techniques if the position of the tumour target can be accurately determined and an active irradiation system can follow the movements of the treated organ. To achieve this, two approaches have been considered. One uses feedback systems that redirect the moving beam during scanning, while the other uses the multiple “repainting” of the target to avoid the local delivery of larger or smaller doses than predicted. In the former, the online motion correction can be done in 3D, using the scanning system for the lateral correction and a fast passive absorber for the depth correction. Experiments at GSI with a phantom showed that the homogeneity and the steep gradients can be preserved to 95% compared with static target irradiation. PSI will pursue the latter approach with proton beams at the PROSCAN project.
Scientists at KEK and TERA have proposed two types of fast cycling accelerators, better suited than cyclotrons and synchrotrons to treating moving organs. They are, respectively, the fixed field alternating gradient accelerator (a mixture of a cyclotron and a synchrotron) and the cyclinac (the combination of a low-energy high-current cyclotron and a high-frequency linac), both of them suitable for proton and carbon-ion therapies. It will take a few years to see what the best solution is both economically and technically.
Without waiting for these developments, industry has shown interest in the upcoming market of hadron therapy, proposing solutions based on synchrotrons and cyclotrons. Five companies already sell proton-therapy units. In the heavy-ion market Mitsubishi has designed a micro-HIMAC, a synchrotron for combined proton and carbon therapy, while Siemens Particle Therapy offers a combined proton/carbon facility on the basis of exclusive licences of the GSI patents and know-how. At present a commercial company is discussing the licensing of the PIMMS/CNAO synchrotron design with the CNAO Foundation. Moreover scientists at the INFN Laboratori Nazionali del Sud in Catania have designed a 300 MeV/u superconducting cyclotron that accelerates hydrogen molecules and carbon ions, allowing treatment with protons of all tumours as well as treatment with carbon ions of tumours located at a water depth of about 15 cm. The Ion Beam Application company in Belgium has transformed this design into a commercial product. The recent interest of industrial companies in ion therapy indicates its large potential, which has its roots in the instruments developed for fundamental research in subatomic physics.
Further reading
U Amaldi and G Kraft 2005 Rep. Prog. Phys. 68 1861. (This paper can be found at www.tera.it/ise/attach/DFILE/639/ROP.pdf.)
L Badano et al. 1999 Proton-Ion Medical Machine Study (PIMMS) Part I CERN/PS 1999-010 DI.
L Badano et al. 2000 Proton-Ion Medical Machine Study (PIMMS) Part II CERN/PS 2000-007 DR.
“Carbon-ion therapy, proceedings of the HPCBM and ENLIGHT meetings held in Baden (Sept. 2002) and in Lyon (Oct. 2003)” 2004 Rad. and Oncol. 73 Suppl. 2 1.
T Haberer et al. 1993 Nucl. Inst. Meth. Phys. Res. A 330 296.
G Kraft 2000 Prog. Part. Nucl. Phys. 45 473
E Pedroni et al. 2004 Z. Med. Phys. 14 25.
S Rossi 2006 Proc. EPAC 2006 3631.
R R Wilson 1946 Radiology 47 487.
R R Wilson 1997 foreword to Advances in Hadron therapy eds. U Amaldi, B Larsson and Y Lemoigne (Elsevier, Amsterdam) ix.