PARTNER – an innovative training project in the field of hadron therapy.

Image credit: PARTNER.
The Particle Training Network for European Radiotherapy (PARTNER) was established in 2008 to train young biologists, engineers, radio-oncologists and physicists in the various aspects of hadron therapy. This deceptively simple statement hides a vision that was truly innovative when the project started: to offer a multidisciplinary education in this cutting-edge discipline to train a future generation of experts who would be aware of the different scientific and technological challenges and move the field forward. PARTNER went on to provide research and training opportunities for 29 young scientists from a variety of backgrounds and countries, between 2008 and 2012. The publication of selected papers from PARTNER in the Journal of Radiation Research offers the opportunity to assess the research outcomes of the project.
As a Marie Curie Initial Training Network (ITN) within the European Union’s 7th Framework Programme (FP7), PARTNER was naturally focused on education, with a training programme encompassing science, technology and transferable skills (CERN Courier March 2010 p27). At the same time, the young scientists became engaged in research on a variety of topics from radiobiology to motion monitoring techniques, dosimetry, accelerators, computing and software tools. All of the research projects shared a focus on the impacts of clinical application, and many brought significant advances to the field.
Ingenious technologies
A key technology area is the development of affordable hadron-therapy installations. The next generation of accelerators should be smaller and less expensive. At the same time, they should allow fast, active energy modulation and have a high repetition rate, so that moving organs can be treated appropriately in reasonable time. PARTNER contributed to the design for the CArbon BOoster for Therapy in Oncology (CABOTO) – a compact, efficient high-frequency linac to accelerate C6+ ions and H2+ molecules from 150 to 410 MeV/u in about 24 m.
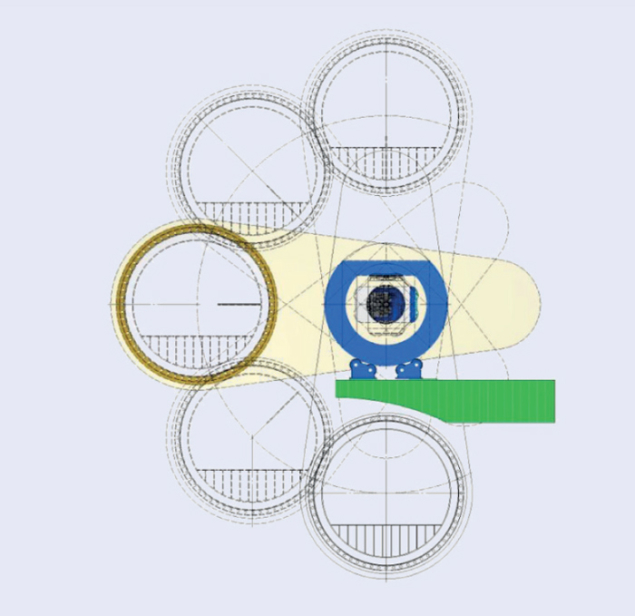
Image credit: ULICE.
Gantries – the magnetic structures that bring particle beams onto the patient at the desired angle – are a major issue in the construction of carbon-ion facilities. The only existing carbon-ion gantry is installed at the Heidelberg Ion-Beam Therapy Center (HIT). It is a fixed, isocentric gantry 6.5 m tall and 25 m long, with a total weight of 600 tonnes. A design study supported by PARTNER and the FP7 project ULICE (CERN Courier December 2011 p37) proposed an innovative solution based on both the gantry and the treatment room being mobile. The isocentric gantry consists of a 90° bending dipole that rotates around the axis of the beam entrance, while the treatment room can move ±90°, thanks to an arrangement that keeps the floor of the room horizontal – like the cabin in a panoramic wheel (see figure). This design reduces the weight and dimensions of the gantry greatly and hence the overall cost.
Clever solutions are also needed to ensure the correct positioning of the patient for treatment. This is particularly important in the case of tumours that change position as organs move – for example, when the patient breathes. A standard technique to reposition the patient accurately at each treatment session involves the implantation of radiographically visible fiducial markers. These markers must not introduce imaging artefacts or perturb the dose delivery process. In particle therapy, however, the interaction of the therapeutic beam with the markers can have a significant impact on the treatment. In this context, PARTNER conducted a study at the treatment set-up at HIT to compare a range of commercially available markers of different materials, shapes and sizes. Some of the markers offered promising results and will soon be used in clinical routine, but the study highlighted that markers should be chosen carefully, taking into account both the tumour localization and the irradiation strategy.
The combination of image guidance with a mask-immobilization system was also investigated at HIT on patients with head-and-neck, brain and skull-base tumours. The study demonstrated that, for the same immobilization device, different imaging verification protocols translate into important differences in accuracy.

Image credit: PARTNER.
At the National Centre for Oncological Treatment (CNAO) in Pavia, PARTNER researchers carried out a comparative analysis of in-room imaging versus an optical tracking system (OTS) for patient positioning. The results showed that while the OTS cannot replace the in-room imaging devices fully, the preliminary OTS correction can greatly support the refinement of the patient set-up based on images, and provide a secondary, independent verification system for patient positioning.
State-of-the-art techniques are also needed for treatment planning – the tool that allows medical physicists to translate the dose prescribed by the oncologists into the set-up parameters for the beam. A PARTNER research project developed a novel Monte Carlo treatment-planning tool for hadron therapy, suitable for treatments delivered with the pencil-beam scanning technique. The tool allows the set-up of single and multiple fields to be optimized for realistic conditions for patient treatment, and also allows dosimetric quality assurance to be performed. Another study led to an accurate parameterization of the lateral dose spread for scanned proton and carbon-ion beams, which is currently in clinical use at HIT and CNAO.
Set-up errors and organ motion can influence the dose distribution during a treatment session. To deal with these potential variations, additional margins are applied to the tumour target, forming the so-called planning target volume (PTV). This procedure ensures that the tumour is irradiated entirely, but inevitably increases the dose delivered to the surrounding healthy tissues. PARTNER researchers studied the generation of a patient-specific PTV from multiple images and were able to achieve satisfactory control of possible target variations, with no significant increase in the dose delivered to organs at risk.
The great attraction of hadron therapy is the possibility of a precisely tailored dose distribution, which allows tumour cells to be hit while sparing the healthy tissues. Sophisticated measurements are needed to verify the actual dose delivered in a specific beam set-up, and air-filled ionization chambers are extensively used in this context. The conversion of data from the ionization chambers into standard dosimetric quantities employs a quality factor that accounts for the specificity of the beam. The ratio of water-to-air stopping power is one of the main components of this quality factor and – in the case of carbon-ion beams – its biggest source of uncertainty. PARTNER researchers developed a fast computational method to determine this stopping-power ratio, with results that were in good agreement with full Monte Carlo calculations.

Image credits: PARTNER.
Faster calculation methods are essential to re-compute the treatment plan quickly when needed, but they should not reduce the accuracy of the treatment planning. The PARTNER studies also demonstrated that a chamber-specific correction could be implemented in the treatment planning, bringing a small improvement to the overall accuracy of the verification of the plan.
Combining treatment modalities has become a standard approach in oncology, and it is important to understand how hadron therapy can fit into these combined treatment schemes. Within the PARTNER framework, three emerging treatment modalities were compared: volumetric-modulated arc therapy (VMAT), intensity- modulated proton beam therapy (IMPT) and intensity-modulated carbon-ion beam therapy (IMIT). Their combinations were also evaluated. The results clearly showed a better dose distribution in the case of combined treatments, but their actual clinical benefit remains to be demonstrated.
Biological factors
In the biological field, studies were performed to understand better the impact of hypoxia – oxygen deprivation – on cell survival, for various types of radiation therapy. Hypoxia is well known as one of the major reasons for the resistance of tumour cells to radiation. It also enhances the risk of metastatic formations. Understanding radioresistance is a key factor for more effective cancer therapy that will minimize local recurrences. Different levels of oxygen deprivation were studied, from intermediate hypoxia to total oxygen deprivation or anoxia. Cells irradiated under chronic anoxia turned out to be more sensitive to radiation than those under acute anoxia. Measurements also suggested that ions heavier than carbon could bring additional advantages in therapeutic irradiation, in particular for radioresistant hypoxic tumour regions.
The initial clinical experience at the CNAO facility provided the opportunity to study toxicity and quality of life for patients under the protocols approved by the Italian Health Ministry, namely for chordoma and chondrosarcoma. The preliminary results showed that all patients completed their treatment with no major toxicities and without interruptions, and that proton therapy did not affect their quality of life adversely. The assessment of quality of life in patients with these tumours is so far unique, as no other study of this kind has been published.
Side effects such as toxicity are an integral part of the information that determines the appropriate choice of treatment. Realistic, long-term data on such effects are difficult to obtain, mainly because of the limited duration of medical studies, so decision-making processes in medicine rely increasingly on modelling and simulation techniques. One of the PARTNER research projects focused on the implementation of a general Markov model for the analysis of side effects in radiotherapy, and developed a specific language to encode the medical understanding of a disease in computable definitions. The proposed method has the potential to automate the generation of Markov models from existing data and to be applicable to many similar decision problems.
Making optimal use of the available resources is a major challenge for the hadron-therapy community, with secure data sharing at the heart of the problem. The Hadron therapy Information Sharing Prototype (HISP) was developed within PARTNER to provide a gateway to patient information that is distributed in many hospital databases, and to support patient follow-up in multicentre clinical studies. HISP demonstrates a range of different and important features, and uses open-source software components that are important for the platform’s sustainable extension and potential for adoption.
The PARTNER network made important contributions to key research areas connected to hadron therapy, geared towards the optimization of this option for cancer treatment. A unique multidisciplinary training portfolio allowed more than 90% of the PARTNER scientists to find positions soon after the end of the project, thanks also to the expertise acquired at the most advanced European hadron-therapy centres and to the networking opportunities provided by the ITN. The medical doctors from India and Singapore went back to their countries and hospitals, while most of the other researchers are now working in hadron-therapy facilities in Europe, the US and Japan. The specific goal of training experts for upcoming and operational facilities was therefore successfully met, and the researchers ensure that the network lives on, wherever they are in the world.
• The PARTNER project was funded by the European Commission within the FP7 People (Marie Curie) Programme, under Grant Agreement No 215840.