Accelerator technology is poised to unleash new treatment modes in more compact ways.
Image credit: MedAustron
Last September the TERA Foundation – dedicated to the study and development of accelerators for particle therapy – celebrated its 25th anniversary. Led by visionary Italian physicist Ugo Amaldi, TERA gathered and trained hundreds of brilliant scientists who carried out research on accelerator physics. This culminated in the first carbon-ion facility for hadron therapy in Italy, and the second in Europe: the National Centre for Cancer Hadron Therapy (CNAO), located in Pavia, which treated its first patient in 2011.
The forerunner to CNAO was the Heidelberg Ion-Beam Therapy Centre (HIT) in Germany, which treated its first patient in 2009 following experience accumulated over 12 years in a pilot project at GSI near Darmstadt. After CNAO came the Marburg Ion-Beam Therapy Centre (MIT) in Germany, which has been operational since 2015, and MedAustron in Wiener Neustadt, Austria, which delivered its first treatment in December 2016.
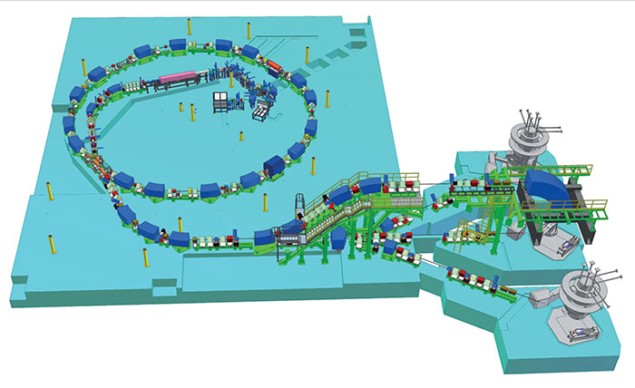
CNAO.
While conventional radiotherapy based on beams of X-rays or electrons is already widespread worldwide, the treatment of cancer with charged particles has seen significant growth in recent years. The use of proton beams in radiation oncology was first proposed in 1946 by Robert Wilson, a student of Ernest Lawrence and founding director of Fermilab. The key advantage of proton beams over X-rays is that the absorption profile of protons in matter exhibits a sharp peak towards the end of their path, concentrating the dose on the tumour target while sparing healthy tissues. Following the first treatment of patients with protons at Lawrence Berkeley Laboratory in the US in 1954, treatment centres in the US, the former USSR and Japan gradually appeared. At the same time, interest arose around the idea of using heavier ions, which offer a higher radio-biological effectiveness and, causing more severe damage to DNA, can control the 3% of all tumours that are radioresistant both to X-rays and protons. It is expected that by 2020 there will be almost 100 centres delivering particle therapy around the world, with more than 30 of them in Europe (see “The changing landscape of cancer therapy”).
Europe entered the hadron-therapy field in 1987, when the European Commission launched the European Light Ion Medical Accelerator (EULIMA) project to realise a particle-therapy centre. The facility was not built in the end, but interest in the topic continued to grow. In 1991, together with Italian medical physicist Giampiero Tosi, Amaldi wrote a report outlining the design of a hospital facility for therapy with light ions and protons to be built in Italy. One year later, the pair established the TERA Foundation to raise the necessary funding to employ students and researchers to work on the project. Within months, TERA could count on the work of about 100 physicists, engineers, medical doctors and radiobiologists, who joined forces to design a synchrotron for particle therapy and the beamlines and monitoring systems necessary for its operation.
Ten years of ups and downs followed, during which TERA scientists developed three designs for a proton-therapy facility initially to be built in Novara, then in the outskirts of Milan and finally in Pavia. Political, legislative and economic issues delayed the project until 2001 when, thanks to the support of Italian health minister and oncologist Umberto Veronesi, the CNAO Foundation was created. The construction of the actual facility began four years later.
“We passed through hard times and we had to struggle, but we never gave up,” says Amaldi. “Besides, we kept ourselves busy with improving the design of our accelerator.”

Image credit: CNAO.
Introducing PIMMS
Meanwhile, in Austria, experimental physicist Meinhard Regler had launched a project called Austron – a sort of precursor to the European Spallation Source. In 1995, together with the head designer – accelerator physicist Phil Bryant – he proposed the addition of a ring to the facility that would be used for particle therapy (and led to the name of the project being changed to MedAustron). Amaldi, Regler and Bryant then decided to work on a common project, and the “Proton-Ion Medical Machine Study” (PIMMS) was created. Developed at CERN between 1996 and 2000 under the leadership of Bryant and with the collaboration of several CERN physicists and engineers, PIMMS aimed to be a toolkit for any European country interested in building a proton–ion facility for hadron therapy. Rather than being a blueprint for a final facility on a specific site, it was an open study from which different parts could be included in any hadron-therapy centre according to its specific needs.
The design of CNAO itself is based on the PIMMS project, with some modifications introduced by TERA to reduce the footprint of the structure. The MedAustron centre, designed in the early 2000s, also drew upon the PIMMS report. Built between 2011 and 2013, with the first beam extracted by the synchrotron in autumn 2014, MedAustron received official certification as a centre for cancer therapy in December 2016 and, a few days after, treated its first patient. “In the past few years we have worked hard to provide the MedAustron trainees with a unique opportunity to acquire CERN’s know-how in the diverse fields of accelerator design, construction and operation,” says Michael Benedikt of CERN, who led the MedAustron accelerator project. Synergies with other CERN projects were also created, he explains. “The vacuum control system built for MedAustron was successfully used in the Linac4 test set-up, while in the synchrotron a novel radiofrequency system that was jointly developed for the CERN PS Booster and MedAustron is used. The synchrotron’s power converter control uses the same top-notch technology as CERN’s accelerators, while its control system and several of its core components are derived from technologies developed for the CMS experiment.”
Image credit: MedAustron/T Kästenbauer
All the existing facilities using hadrons for cancer therapy are based on circular cyclotrons and synchrotrons. For some years, however, the TERA Foundation has been working on the design of a linear accelerator for hadron therapy. As early as 1993, Amaldi set up a study group, in collaboration with the Italian institutions ENEA and INFN, dedicated to the design of a linac for protons that would run at the same frequency (3 GHz) as the electron linacs used for conventional radiotherapy. The linac could use a cyclotron as an injector, making it a hybrid solution called a cyclinac, which reduces the sizes of both accelerators while allowing the beam energy to be rapidly changed from pulse to pulse by acting on the radiofrequency system of the linac. In 1998 a 3 GHz 1.2 metre-long linac booster (LIBO) was built by a TERA–CERN–INFN collaboration led by retired CERN engineer Mario Weiss, and in 2001 it was connected to the cyclotron of the INFN South Laboratories in Catania where it accelerated protons from 62 MeV to 74 MeV. This was meant to be the first of 10 modules that would kick protons to 230 MeV.
Linear ambition
In 2007 a CERN spin-off company called ADAM (Applications of Detectors and Accelerators to Medicine) was founded by businessman Alberto Colussi to build a commercial high-frequency linac based on the TERA design. Under the leadership of Stephen Myers, a former CERN director for accelerators and technology and initiator of the CERN medical applications office, ADAM is now completing the first prototype. It is called Linac for Image Guided Hadron Therapy (LIGHT), and the full accelerator comprises: a proton source; a novel 750 MHz RF quadrupole (RFQ) – designed by CERN – which takes the particles up to 5 MeV; four side-coupled drift-tube linacs (SCDTL) – designed by ENEA – to accelerate the beam from 5–37.5 MeV; and a different type of accelerating module, called coupled-cavity linac (CCL) – the LIBO designed by TERA – which gives the final kick to the beam from 37.5 to 230 MeV. The complex will be 24 m long, similar to the circumference of a proton synchrotron.
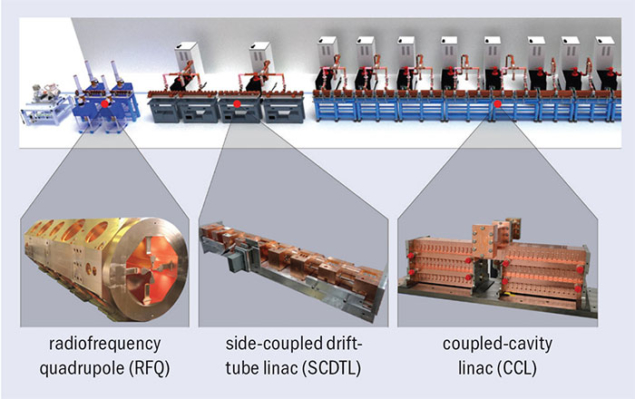
Image credit: AVO-ADAM.
Compared to cyclotrons and synchrotrons, linear accelerators are lighter and potentially less costly because they are modular. Most importantly, they produce a beam much more suited to treat patients, in particular when the tumour is moving, as in the lungs. The machine developed by ADAM is modular in structure to make it easier to maintain and more flexible when it comes to upgrading or customising the system. In addition, thanks to an active longitudinal modulation system, the beam energy can be varied during therapy and thus the treatment depth changed. LIGHT also has a dynamic transversal modulation system, allowing the beam to be rapidly and precisely modulated to “paint the tumour” many times in a short time – in other words, delivering a homogeneous dose to the whole cancerous tissue while minimising the irradiation of healthy organs. The energy variation of cyclotrons and synchrotrons is 20–100 times slower.
“The beauty of the linac is that you can electronically modulate its output energy,” Myers explains. “Since our accelerator is modular, the energy can be changed either by switching off some of the units or by reducing the power in all of them, or by re-phasing the units. Another big advantage of the linac is that it has a small emittance, i.e. beam size, which translates into smaller, lighter and cheaper magnets and allows to have a simpler and lighter gantry as well.” In the last decade, LIBO has inspired other TERA projects. Its scientists have designed a linac booster for carbon ions (while LIBO was only for protons) and a compact single-room facility called TULIP, in which a 7 m-long proton linac is mounted on a rotating gantry.
The new frontier of hadron therapy, however, could be helium ion treatment. Some tests with these ions were done in the past, but the technique still has to be proven. TERA scientists are currently working on a new accelerator for helium ions, says Amaldi. “Helium can bring great benefit to medical treatments: it is lighter than carbon, thus requiring a smaller accelerator, and it has much less lateral scattering than protons, resulting in sharper lateral fall-offs next to organs at risk.” In order to accelerate helium ions with a linac, we need either a longer linac compared to the one used for protons or higher gradients, as demonstrated by high-energy physics research at CERN and elsewhere in Europe. The need for future, compact and cost-effective ion-therapy accelerators is being addressed by a new collaborative design study coordinated by Maurizio Vretenar and Alessandra Lombardi of CERN, dubbed “PIMMS2”. A proposal, which includes a carbon linac, is being prepared for submission to the CERN Medical Application group, potentially opening the next phase of TERA’s impressive journey.





