Peter Rosen looks back at the man who brought his deft chemical touch to the study of neutrinos, and created one of the iconic experiments of the 20th century.
Raymond Davis Jr, discoverer and grand pioneer of the solar-neutrino problem, died on 31 May at the venerable age of 91. In 1968 he discovered the solar-neutrino anomaly and more than three decades later he received the 2002 Nobel Prize in Physics. This followed other experiments based on different techniques, which demonstrated that the anomaly was neither an artifact of his experiment nor an error in the late John Bahcall’s theoretical calculations of the neutrino flux from the Sun; it was indeed a real physical effect. Simultaneously, Davis had shown that the Sun generates its energy by nuclear fusion of hydrogen into helium, and that the electron-type neutrinos created in this process change into other types of neutrino during their eight minute journey to Earth.

By his own account, Davis was drawn to the study of neutrinos out of a sense of adventure. He was invited to join the fledgling Brookhaven National Laboratory in 1948 and asked the chairman of the chemistry department about his duties. To his “surprise and delight” Davis was told to choose his own project. In the library he found a review article on neutrinos by H R Crane that clearly indicated that the field was wide open for exploration and rich in problems. Here was the path, leading he knew not where, which would enable Davis to follow his goal of studying nuclear physics using the techniques of physical chemistry.

His vehicle was a nuclear reaction suggested by Bruno Pontecorvo in 1946: a neutrino captured by a specific isotope of chlorine produces an electron and a radioactive isotope of argon. Sources of chlorine were plentiful and cheap, and argon, a noble gas, could easily be extracted from a chlorine solution. Davis counted the atoms of the argon isotope by observing the decay back to chlorine. Over the years, he tested and refined the method so that it became totally reliable as a procedure for measuring even a tiny number of argon atoms produced in Pontecorvo’s chlorine reaction.
The only copious sources of low-energy neutrinos are nuclear reactors and the Sun. Reactors produce antineutrinos from the beta-decay of heavy nuclei following the fission of uranium, while the Sun produces neutrinos in the fusion of hydrogen nuclei into helium. When Davis was beginning his experiments, the distinction between neutrino and antineutrino was not well understood and there was a serious possibility that they were identical particles. It was therefore natural for him to use reactors as the neutrino source. In fact, he worked at the Savannah River reactor at the time when Clyde Cowan and Fred Reines were performing their Nobel prize-winning experiment there using inverse beta-decay as their signal. Whereas they obtained a positive result, making the first observation of the antineutrino, Davis obtained a null result with the chlorine reaction, indicating a distinction between neutrino and antineutrino.
Davis then turned his attention to detecting neutrinos from the Sun and recognized that it was necessary to go deep underground to avoid cosmic-ray backgrounds. He also realized that observing neutrinos from the Sun would be a way of demonstrating that it generates its energy via nuclear fusion. In the early 1950s, it was known that the proton–proton fusion chain did not produce neutrinos of sufficient energy to reach the threshold for the chlorine reaction, but by the end of the decade new discoveries in nuclear physics suggested that there were additional fusion chains that produced neutrinos well above the threshold. Davis began to collaborate with Bahcall to design an experiment to observe these neutrinos.
By 1964, they were ready to propose an experiment and they published side-by-side papers in Physical Review Letters. Bahcall combined the standard model of the Sun with the relevant nuclear physics to calculate the energies and fluxes of the various branches of the solar-neutrino spectrum. Davis described an experiment to observe the higher-energy neutrinos using a 100,000 gallon tank of cleaning fluid (perchlorethylene) located deep underground. Bahcall tells an interesting story of how Davis persuaded Maurice Goldhaber, then director of Brookhaven National Laboratory, to support the experiment. Knowing that Goldhaber was very sceptical of astrophysical calculations, Davis instructed Bahcall not to mention the solar astrophysics, but to emphasize instead the novel nuclear physics involved in the chlorine reaction. Goldhaber loves a clever idea, having generated many himself, and so, as Davis predicted, he responded positively to Davis’ request.
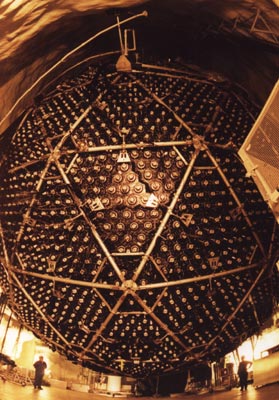
In 1968, Davis reported that the first measurements from the experiment carried out 4850 ft underground in the Homestake gold mine in Lead, South Dakota, yielded a solar-neutrino capture rate approximately a third of that predicted by Bahcall and G Shaviv. This “socially unacceptable result”, as Bahcall later described it, caused widespread concern among both physicists and astrophysicists. Some thought that the problem lay with the experiment, others with the theory and a few with the neutrino. In the end the third option turned out to be correct, but it required an experimental and theoretical odyssey that lasted three and a half decades and ranged all over the world, from the mine in South Dakota to other mines and laboratories deep inside mountains in Japan, Russia and Italy, and finally to a nickel mine at Sudbury in northern Ontario, Canada.
The experiments themselves were remarkable in their uniqueness. Davis’ experiment required a 100,000 gallon tank specially built by the Chicago Bridge and Iron Company, and it took 10 railroad tank cars of cleaning fluid to fill it. The detector in Japan was the size of a 10 storey building. It was filled with extremely pure and continuously purified water and was surrounded by 11,000 20-inch phototubes. The neutrino detector in Russia, located deep inside the Caucasus Mountains, contained the world’s total supply of gallium, about 60 tonnes. It took two years to produce an additional 30 tonnes of gallium for an independent experiment under the Gran Sasso Mountain of central Italy. The experiment in Canada, originally proposed by the late Herbert Chen many years ago, would not have been possible without the loan of a kilotonne of heavy water from the Canadian government.
Throughout this journey, Davis continued his role as grand pioneer of the solar-neutrino problem. He kept the chlorine experiment going long after he retired from Brookhaven National Laboratory in 1984. He helped to develop the radiochemical experiment based on gallium, sensitive to the most-copious and lowest-energy solar neutrinos from the proton–proton chain, and he followed all the latest developments with a keen interest. Ultimately, at the beginning of the 21st century, the heavy-water experiment at Sudbury measured both the total highest-energy neutrino flux from the Sun and its electron–neutrino component. It confirmed Davis’ original results as well as Bahcall’s theoretical calculations, and it crowned Davis’ claim on the Nobel prize.
Davis had a truly inquiring and adventurous mind. “When I began my work, I was intrigued by the idea of learning something new,” he once said. “The interesting thing about doing new experiments is that you never know what the answer is going to be!” He was a scientist of great integrity and modesty: what mattered to him was not himself, but the science in which he was involved. Whenever colleagues asked questions or offered criticisms of his experiment, he would always devise new tests to check their ideas and make any necessary corrections. But he also had a wry sense of humour: when asked at a conference in the 1970s how much his experiment cost, he replied: “Ten minutes time on commercial television.”
Through his persistence, integrity and humility Davis spawned a revolution in neutrino physics and gave us a beautiful example of how different sciences can help one another to make fundamental discoveries. He stands as a model for all aspiring scientists to emulate.








