CERN-MEDICIS will use a proton beam from ISOLDE to develop non-conventional isotopes for medical diagnostics and therapy.
Résumé
Le CERN produira des radio-isotopes pour la médecine
Le lien entre les communautés des accélérateurs et de la médecine remonte à presque 50 ans. Aujourd’hui, alors que les physiciens développent la nouvelle génération de machines pour la recherche, les médecins imaginent de nouvelles méthodes pour diagnostiquer et traiter les maladies neurodégénératives et les cancers. Le projet MEDICIS du CERN vise à développer de nouveaux isotopes pouvant être utilisés à la fois comme agents de diagnostic et pour la curiethérapie ou la radiothérapie interne avec source non scellée, pour le traitement de cancers du cerveau ou du pancréas non opérables et d’autres formes de cette maladie. L’installation, dont l’idée a germé en 2010 et qui sera opérationnelle en 2017, utilise un faisceau de protons et l’installation de faisceaux d’ions radioactifs ISOLDE pour produire des isotopes médicaux. Ces isotopes seront d’abord destinés à des hôpitaux et des centres de recherche en Suisse, puis progressivement à d’autres laboratoires en Europe et ailleurs dans le monde.

Image credit: Yury Gavrikov.
Accelerators and their related technologies have long been developed at CERN to undertake fundamental research in nuclear physics, probe the high-energy frontier or explore the properties of antimatter. Some of the spin-offs of this activity have become key to society. A famous example is the World Wide Web, while another is medical applications such as positron emission tomography (PET) scanner prototypes and image reconstruction algorithms developed in collaboration between CERN and Geneva University Hospitals in the early 1990s. Today, as accelerator physicists develop the next-generation radioactive beam facilities to address new questions in nuclear structure – in particular HIE-ISOLDE at CERN, SPIRAL 2 at GANIL in France, ISOL@Myrrha at SCK•CEN in Belgium and SPES at INFN in Italy – medical doctors are devising new approaches to diagnose and treat diseases such as neurodegenerative disorders and cancers.
The bridge between the radioactive-beam and medical communities dates back to the late 1970s, when radioisotopes collected from a secondary beam at CERN’s Isotope mass Separator On-Line facility (ISOLDE) were used to synthesise an injectable radiopharmaceutical in a patient suffering from cancer. 167Tm-citrate, a radiolanthanide associated to a chelating chemical, was used to perform a PET image of a lymphoma, which revealed the spread-out cancerous tumours. While PET became a reference protocol to provide quantitative imaging information, several other pre-clinical and pilot clinical tests have been performed with non-conventional radioisotopes collected at radioactive-ion-beam facilities – both for diagnosis and for therapeutic applications.

Image credit: Yury Gavrikov.
Despite significant progress made in the past decade in the field of oncology, however, the prognosis of certain tumours is still poor – particularly for patients presenting advanced glioblastoma multiforme (a form of very aggressive brain cancer) or pancreatic adenocarcinoma. The latter is a leading cause of cancer death in the developed world and surgical resection is the only potential treatment, although many patients are not candidates for surgery. Although external-beam gamma radiation and chemotherapy are used to treat patients with non-operable pancreatic tumours, and survival rates can be improved by combined radio- and chemotherapy, there is still a clear need for novel treatment modalities for pancreatic cancer.
A new project at CERN called MEDICIS aims to develop non-conventional isotopes to be used as a diagnostic agent and for brachytherapy or unsealed internal radiotherapy for the treatment of non-resectable brain and pancreatic cancer, among other forms of the disease. Initiated in 2010, the facility will use a proton beam at ISOLDE to produce isotopes that first will be destined for hospitals and research centres in Switzerland, followed by a progressive roll-out to a larger network of laboratories in Europe and beyond. The project is now approaching its final phase, with start-up foreseen in June 2017.
A century of treatment
The idea of using radioisotopes to cure cancer was first proposed by Pierre Curie soon after his discovery of radium in 1898. The use of radium seduced many physicians because the penetrating rays could be used superficially or be inserted surgically into the body – a method called brachytherapy. The first clinical trials took place at the Curie Institute in France and at St Luke’s Memorial Hospital in New York at the beginning of the 20th century, for the treatment of prostate cancer.

Image credit: Yury Gavrikov.
A century later, in 2013, a milestone was met with the successful clinical trials of 223Ra in the form of the salt-solution RaCl2, which was injected into patients suffering from prostate cancers with bone metastasis. The positive effect on patient survival was so clear in the last clinical validation (so-called phase III), that the trial was terminated prematurely to allow patients who had received a placebo to be given the effective drug. Today, the availability of new isotopes, medical imagery, robotics, monoclonal antibodies and a better understanding of tumour mechanisms has enabled progress in both brachytherapy and unsealed internal radiotherapy. Radioisotopes can now be placed closer to and even inside the tumour cells, killing them with minimal damage to healthy tissue.
CERN-MEDICIS aims to further advance this area of medicine. New isotopes with specific types of emission, tissue penetration and half-life will be produced and purified based on expertise acquired during the past 50 years in producing beams of radioisotope ions for ISOLDE’s experimental programme. Diagnosis by single photon emission computed tomography (SPECT), a form of scintigraphy, covers the vast majority of worldwide isotope consumption based on the gamma-emitting 99mTc, which is used for functional probing of the brain and various other organs. PET protocols are increasingly used based on the positron emitter 18F and, more recently, a 68Ga compound. Therapy, on the other hand, is mostly carried out with beta emitters such as 131I, more recently with 177Lu, or with 223Ra for the new application of targeted alpha therapy. Other isotopes also offer clear benefits, such as 149Tb, which is the lightest alpha-emitting radiolanthanide and also combines positron-emitting properties.
Driven by ISOLDE
With 17 Member States and an ever-growing number of users, ISOLDE is a dynamic facility that has provided beams for around 300 experiments at CERN in its 50 year history. It allows researchers to explore the structure of the atomic nucleus, study particle physics at low energies, and provides radioactive probes for solid-state and biophysics. Through 50 years of collaboration between the technical teams and the users, a deep bond has formed, and the facility evolves hand-in-hand with new technologies and research topics.

Image credit: Yury Gavrikov.
CERN MEDICIS is the next step in this adventure, and the user community is joining in efforts to push the development of the machine in a new direction. The project was initiated six years ago by a relatively small collaboration involving CERN, KU Leuven, EPFL and two local University Hospitals (CHUV in Lausanne and HUG in Geneva). One year later, in 2011, CERN decided to streamline medical production of radioisotopes and to offer grants dedicated to technology transfer. While the mechanical conveyor system to transport the irradiated targets was covered by such a grant, the construction of the CERN MEDICIS building began in September 2013. The installation of the services, mass separator and laboratory is now under way.
At ISOLDE, physicists direct a high-energy proton beam from the Proton Synchrotron Booster (PSB) at a target. Since the beam loses only 10% of its intensity and energy on hitting the target, the particles that pass through it can still be used. For CERN-MEDICIS, a second target therefore sits behind the first and is used for exotic isotope generation. Key to the project is a mechanical system that transports a fresh target and its ion source into one of the two ISOLDE target-stations’ high resolution separator (HRS) beam dump, irradiates it with the proton beam from the PSB to generate the isotopes, then returns it to the CERN-MEDICIS laboratory. The system was fully commissioned in 2014 under proton-beam irradiation with a target that was later used to produce a secondary beam, thus validating the full principle. A crucial functional element was still missing: the isotope mass separator, along with its services and target station. Coincidentally, however, CERN MEDICIS started just as the operation of KU Leuven’s isotope-separation facility ended, and a new lease of life could therefore be given to its dipole magnet separator, which was delivered to CERN earlier this year for testing and refurbishment.
A close collaboration is growing at MEDICIS centred around the core team at CERN but involving partners from fundamental nuclear physics, material science, radiopharmacy, medical physics, immunology, radiobiology, oncology and surgery, with more to come.
Training network
With such an exceptional tool at hand, and based on growing pre-clinical research experiments performed at local university hospitals, in 2014 a H2020 Innovative Training Network was set up by CERN to ensure MEDICIS is fully exploited. This “Marie Skłodowska-Curie actions” proposal was submitted to the European Commission entitled MEDICIS-Promed, which stands for MEDICIS-produced radioisotope beams for medicine. The goal of this 14-institution consortium is to train a new generation of scientists to develop systems for personalised medicine combining functional imaging and treatments based on radioactive ion-beam mass separation. Subsystems for the development of new radiopharmaceuticals, isotope mass separators at medical cyclotrons, and of mass-separated 11Carbon for PET-aided hadron therapy are to be specifically developed to treat ovarian cancer. Pre-clinical experiments have already started, with the first imaging studies ever done with these exotic radioisotopes. For this, a specific ethical review board has been implemented within the consortium and is chaired by independent members.
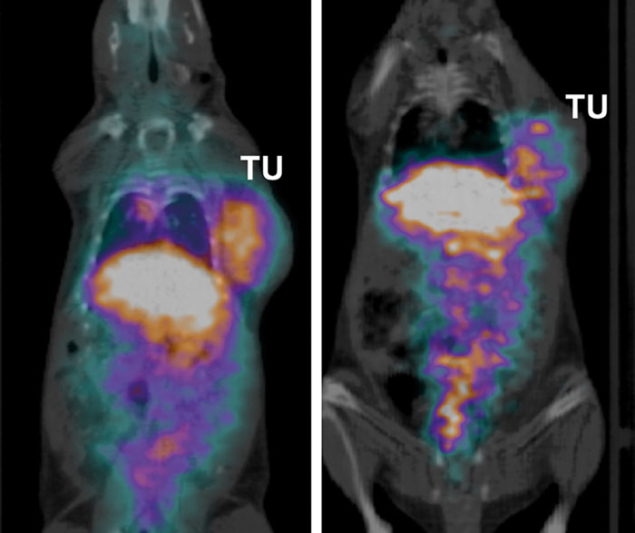
Image credit: F Cicone et al., MEDICIS-Promed.
With the MEDICIS facility entering operation next year, an increasing range of innovative isotopes will progressively become accessible. These will be used for fundamental studies in cancer research, for new imaging and therapy protocols in cell and animal models, and for pre-clinical trials – possibly extended to early phase clinical studies up to Phase I trials. During the next few years, 500 MBq isotope batches purified by electromagnetic mass separation combined with chemical methods will be collected on a weekly basis. This is a step increase in production to make these innovative isotopes more available to biomedical research laboratories, compared with the present production of a few days per year in a facility such as ISOLDE.
Staged production
During its initial stage in 2017, only low-Z materials, such as titanium foils and Y2O3 ceramics, will be used as targets. From these, we will produce batches of several hundred MBq of 44,47Sc and 61,64Cu. In the second stage, tentatively scheduled for 2018, we will use targets from the nuclei of higher atomic numbers, such as tantalum foils, to reach some of the most interesting terbium and lanthanide isotopes. In a final phase in 2018, we foresee the use of uranium and thorium targets to reach an even wider range of isotopes and most of the other alpha-emitters.

Selected isotopes will first be tested in vitro for their capacity to destroy glioblastoma or pancreatic adenocarcinoma or neudoendocine tumour cells, and in vivo by using mouse models of cancer. We will also test the isotopes for their direct effect on tumours and when they are coupled to peptides with tumour-homing capacities. New delivery methods for brachytherapy using stereotactic, endoscopic ultrasonographic-guided or robotic-assisted surgery will be established in large-animal models.
Moreover, this new facility marks the entrance of CERN into the era of theranostics. This growing oncological field allows nuclear-medicine physicians to verify and quantify the presence of cellular and molecular targets in a given patient with the diagnostic radioisotope, before treating the disease with the therapeutic radioisotope. The prospect of a dedicated facility at CERN for the production of innovative isotopes, together with local leading institutes in life and medical sciences and a large network of laboratories, makes this an exciting scientific programme in the coming years.