In the June 2005 issue of CERN Courier (the last to be published jointly in French and English), David Townsend – who began his pioneering work in PET imaging 30 years earlier while a staff member at CERN – talked to Beatrice Bressan about the development and clinical impact of this field.

David Townsend is a professor in the Department of Medicine, University of Tennessee Medical Center in Knoxville, Tennessee (TN). The winner of the 2004 Clinical Scientist of the Year Award from the Academy of Molecular Imaging, he is an internationally renowned researcher with 30 years’ experience as a physicist working in the field of positron emission tomography (PET). Townsend began his eight years at CERN in 1970. While working at the Cantonal Hospital in Geneva from 1979 to 1993, he recognised the importance of combining the functionality of PET with that of computed tomography (CT). During that same period, Townsend also worked with Georges Charpak, CERN physicist and 1992 Nobel laureate in physics, on medical applications of Charpak’s multi-wire chambers.
After Townsend moved to Pittsburgh in 1993, his group in the US helped to develop the first combined PET/CT scanner; more than 1000 are now used worldwide to image human cancer. In 1999, Townsend received the Image of the Year Award from the Society of Nuclear Medicine in the US, for an image he produced using the first prototype scanner combining state-of-the art PET with true diagnostic-quality CT.
Current research objectives in instrumentation for PET include advances in PET/CT methodology and the assessment of the role of combined PET/CT imaging for a range of different cancers. The PET/CT combination, pioneered by Townsend and Ron Nutt, CEO and president of CTI Molecular Imaging in Knoxville, TN, is a milestone in these developments, revealing in particular the role of the physicist and engineer in bringing such developments into clinical practice and exploring how they affect patient care.
The past 20 years have seen significant advances in the development of imaging instrumentation for PET. Current high-performance clinical PET scanners comprise more than 20,000 individual detector elements, with an axial coverage of 16 cm and around 15% energy resolution. Can you identify the most important factors that have contributed to this remarkable development in PET?
This impressive progress is due essentially to developments in detector construction, new scintillators, better scanner designs, improved reconstruction algorithms, high-performance electronics and, of course, the vast increase in computer power, all of which have been achieved without an appreciable increase in the selling price of the scanners.
The PET/CT image is one of the most exciting developments in nuclear medicine and radiology, its significance being the merging not simply of images but of the imaging technology. Why is the recent appearance of combined PET and CT scanners that can simultaneously image both anatomy and function of particular importance?
Initial diagnosis and staging of tumours are commonly based on morphological changes seen on CT scans. However, PET can differentiate malignant tissue from benign tissue and is a more effective tool than CT in the search for metastases. Clearly, valuable information can be found in both, and by merging the two it is possible now to view morphological and physiological information in one fused image. To acquire the PET/CT image, a patient passes through the CT portion of the scanner first and then through the PET scanner where the metabolic information is acquired. When the patient has passed through both portions, a merged or fused image can be created.
Let’s take a step back. The history of PET is rich, dynamic and marked by many significant technological achievements. Volumes of books would be required to record the history of PET developments and its birth still remains quite controversial. Could you identify the most important events that have shaped modern PET?
You are indeed correct that the birth of PET is somewhat controversial. One of the first suggestions to use positron-emitting tracers for medical applications was made in 1951 by W H Sweet and G Brownell at Massachusetts General Hospital, and some attempts were made to explore the use of positron-emitting tracers for medical applications in the 1950s. During the late 1950s and 1960s, attempts were made to build a positron scanner, although these attempts were not very successful. After the invention of the CT scanner in 1972, tomography in nuclear medicine received more attention, and during the 1970s a number of different groups attempted to design and construct a positron scanner.
S Rankowitz and J S Robertson of Brookhaven National Laboratory built the first ring tomograph in 1962. In 1975, M Ter-Pogossian, M E Phelps and E Hoffman at Washington University in St Louis presented their first PET tomograph, known as Positron Emission Transaxial Tomograph I (PETT I). Later the name was changed to PET, because the transaxial plane was not the only plane in which images could be reconstructed. In 1979, G N Hounsfield and A M Cormack were awarded the Nobel Prize for Physiology and Medicine in recognition of their development of X-ray CT.
Since the very early development of nuclear-medicine instrumentation, scintillators such as sodium iodide (NaI) have formed the basis for the detector systems. The detector material used in PET is the determining factor in the sensitivity, the image resolution and the count-rate capability.
The only detector of choice in the mid-1970s was thallium-activated NaI – NaI(Tl) – which requires care when manufactured because of its hygroscopic nature. More importantly, it also has a low density and a low effective atomic number that limits the stopping power and efficiency to detect the 511 keV gamma rays from positron annihilation. Which other scintillators have contributed to modern PET tomography?
Thanks to its characteristics, bismuth germanate, or BGO, is the crystal that has served the PET community well since the late 1970s, and it has been used in the fabrication of most PET tomographs for the past two decades. The first actual tomograph constructed that employed BGO was designed and built by Chris Thompson and co-workers at the Neurological Institute in Montreal in 1978.
Although the characteristics of BGO are good, a new scintillator, lutetium oxyorthosilicate (LSO) (discovered by C Melcher, now at CTI Molecular Imaging in Knoxville, TN), is a significant advance for PET imaging. BGO is very dense but has only 15% of the light output of NaI(Tl). LSO has a slightly greater density and a slightly lower effective atomic number, but has five times more light output and is seven times faster than BGO. The first LSO PET tomograph, the MicroPET for small animal imaging, was designed at the University of California in Los Angeles (UCLA) by Simon Cherry and co-workers. The first human LSO tomograph, designed for high-resolution brain imaging, was built by CPS Innovations in Knoxville, TN, and delivered to the Max Planck Institute in February 1999.
What were your key achievements in PET during your career at CERN? Did CERN play a role in its birth?

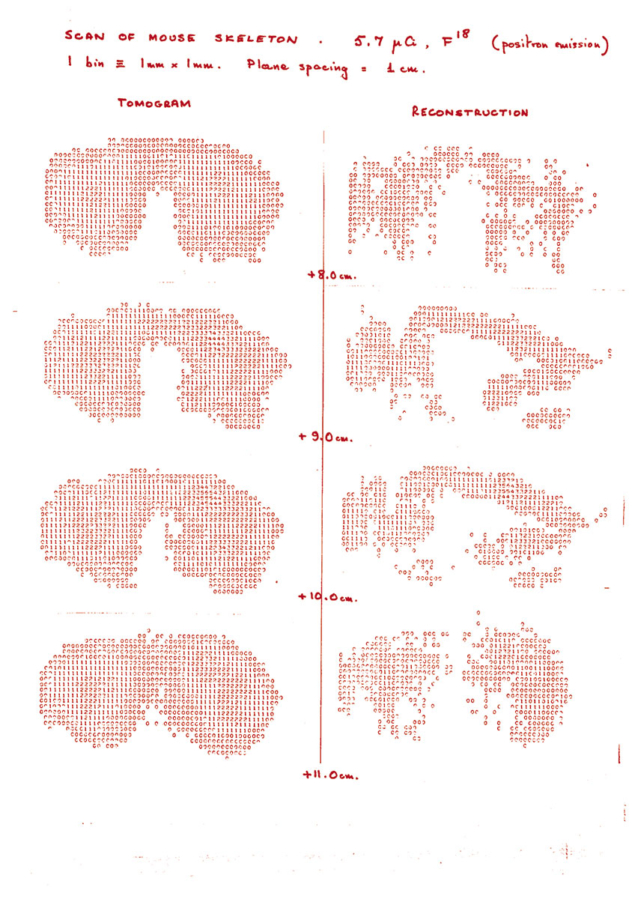
In 1975, I was working at CERN when Alan Jeavons, a CERN physicist, asked me to look at the problem of reconstructing images from PET data acquired on the small high-density avalanche chambers (HIDACs) he had built for another application with the University of Geneva. We got the idea for using the HIDACs for PET because a group in Berkeley and University of California, San Francisco (UCSF) was using wire chambers for PET. I developed some software to reconstruct the data from Jeavons’ detectors, and we took the first mouse image with the participation of radiobiologist Marilena Streit-Bianchi in 1977 at CERN.
The reconstruction methods I developed at CERN were further extended mathematically by Benno Schorr (a CERN mathematician), Rolf Clackdoyle and myself from 1980 to 1982. We used those, and other algorithms developed by Michel Defrise in Brussels and Paul Kinahan in Vancouver, in 1987 and 1988 to reconstruct PET data from the first CTI [Computer Technology and Imaging Inc, renamed CTI Molecular Imaging in June 2002] multi-ring PET scanner installed in London at Hammersmith Hospital. PET was not invented at CERN, but some essential and early work at CERN contributed significantly to the development of 3D PET, and then to a new scanner design, the Advanced Rotating Tomograph (ART).
The prototype of the ART scanner, the Partial Ring Tomograph (PRT), was developed at CERN from 1989 to 1990 by Martin Wensveen, Henri Tochon-Danguy and myself, and evaluated clinically at the Cantonal Hospital within the Department of Nuclear Medicine under Alfred Donath. The ART was a forerunner of the PET part of the combined PET/CT scanner, which has now had a major impact on medical imaging.
What has to happen for us to reach a more highly performing PET/CT combination?
The sensitivity of the PET components must be improved in order to acquire more photons in a given time. That is still a challenge, because the axial coverage of current scanners is only 16 cm, whereas after injection of the radiopharmaceutical, radiation is emitted from everywhere in the patient’s body where the radiopharmaceutical localizes. So, if the detector covered the whole body, the patient could be imaged in one step. However, building such a system would be very expensive.
Do you think it is still possible to have other combinations with other imaging techniques?
Yes, absolutely, but only if there is a medical reason to do it – such a development won’t be driven by advances in technology alone. When we looked at building a PET/CT scanner, we found that most whole-body anatomical imaging for oncology is still performed with CT, whereas in brain and spinal malignancies, anatomical imaging is performed with magnetic resonance (MR).
PET/CT is less technologically challenging than combining PET with MR. PET and CT modalities basically do not interfere with each other, except maybe when they are operated simultaneously within the same gantry. The combined PET/CT scanner provides physicians with a highly powerful tool to diagnose and stage disease, monitor the effects of treatment, and potentially design much better, patient-specific therapies.
What is the actual cost of a PET/CT scanner?
The cost of the highest-performing system is about $2.5 million [€1.98 million], but it may be significantly less if a lower-performance design is adequate for the envisaged application.
- This article was adapted from text in CERN Courier vol. 45, June 2005, pp23–25
Further reading
R Nutt 2002 The History of Positron Emission Tomography Molecular Imaging and Biology 4 11.
D W Townsend 2004 From 3-D Positron Emission Tomography to 3-D Positron Emission Tomography/Computed Tomography: What Did We Learn? Molecular Imaging and Biology 6 275.







