Advanced neutron facilities such as the Institut Laue-Langevin are gearing up to enable a deeper understanding of the structural workings of SARS-CoV-2.

The global scientific community has mobilised at an unprecedented rate in response to the COVID-19 pandemic, beyond just pharmaceutical and medical researchers. The world’s most powerful analytical tools, including neutron sources, harbour the unique ability to reveal the invisible, structural workings of the virus – which will be essential to developing effective treatments. Since the outbreak of the pandemic, researchers worldwide have been using large-scale research infrastructures such as synchrotron X-ray radiation sources (CERN Courier May/June 2020 p29), as well as cryogenic electron microscopy (cryo-EM) and nuclear magnetic resonance (NMR) facilities, to determine the 3D structures of proteins of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which can lead to COVID-19 respiratory disease, and to identify potential drugs that can bind to these proteins in order to disable the viral machinery. This effort has already delivered a large number of structures and increased our understanding of what potential drug candidates might look like in a remarkably short amount of time, with the number increasing each week.
COVID-19 impacted the operation of all advanced neutron sources worldwide. With one exception (ANSTO in Australia, which continued the production of radioisotopes) all of them were shut down in the context of national lockdowns aimed at reducing the spread of the disease. The neutron community, however, lost no time in preparing for the resumption of activities. Some facilities like Oak Ridge National Laboratory (ORNL) in the US have now restarted operation of their sources exclusively for COVID-19 studies. Here in Europe, while waiting (impatiently) for the restart of neutron facilities such as the Institut Laue-Langevin (ILL) in Grenoble, which is scheduled to be operational by mid-August, scientists have been actively pursuing SARS-CoV-2-related projects. Special research teams on the ILL site have been preparing for experiments using a range of neutron-scattering techniques including diffraction, small-angle neutron scattering, reflectometry and spectroscopy. Neutrons bring to the table what other probes cannot, and are set to make an important contribution to the fight against SARS-CoV-2.
Unique characteristics
Discovered almost 90 years ago, the neutron has been put to a multitude of uses to help researchers understand the structure and behaviour of condensed-matter. These applications include a steadily growing number of investigations into biological systems. For the reasons explained below, these investigations are complementary to the use of X-rays, NMR and cryo-EM. The necessary infrastructure for neutron-scattering experiments is provided to the academic and industrial user communities by a global network of advanced neutron sources. Leading European neutron facilities include the ILL in Grenoble, France, MLZ in Garching, Germany, ISIS in Didcot, UK, and PSI in Villigen, Switzerland. The new European flagship neutron source – the European Spallation Source (ESS) – is under construction in Lund, Sweden.
Structural power
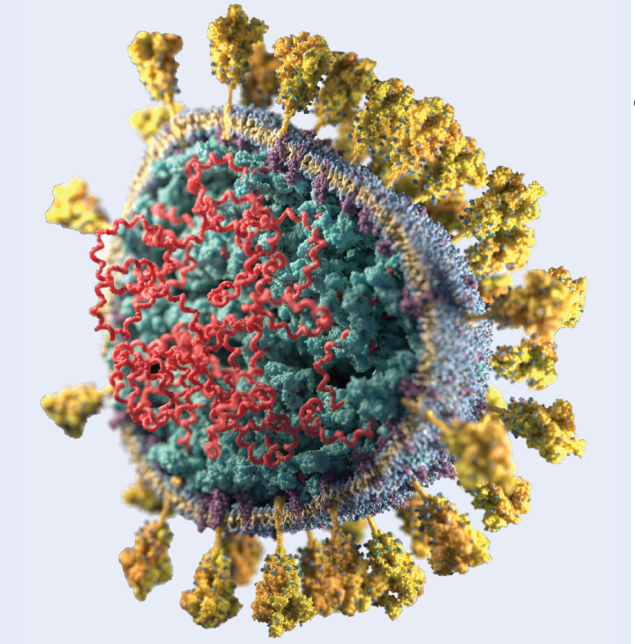
Determining the biological structures that make up a virus such as SARS-CoV-2 (pictured) allows scientists to see what they look like in three dimensions and to understand better how they function, speeding up the design of more effective anti-viral drugs. Knowledge of the structures highlights which parts are the most important: for example, once researchers know what the active site in an enzyme looks like, they can try to design drugs that fit well into the active site – the classic “lock-and-key” analogy. This is also useful in the development of vaccines. Knowledge of the structural components that make up a virus are important since vaccines are often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins.
Neutrons are a particularly powerful tool for the study of biological macromolecules in solutions, crystals and partially ordered systems. Their neutrality means neutrons can penetrate deep into matter without damaging the samples, so that experiments can be performed at room temperature, much closer to physiological temperatures. Furthermore, in contrast to X-rays, which are scattered by electrons, neutrons are scattered by atomic nuclei, and so neutron-scattering lengths show no correlation with the number of electrons, but rather depend on nuclear forces, which can even vary between different isotopes. As such, while hydrogen (H) scatters X-rays very weakly, and protons (H+) do not scatter X-rays at all, with neutrons hydrogen scatters at a similar level to the other common elements (C, N, O, S, P) of biological macromolecules, allowing them to be located. Moreover, since hydrogen and its isotope deuterium (2H/D) exhibit different scattering lengths and signs, this can be exploited in neutron studies to enhance the visibility of specific structural features by substituting one isotope for the other. Examples of this include small-angle neutron scattering (SANS) studies of macromolecular structures that provide low-resolution 3D information on molecular shape without the need for crystallization, and neutron-crystallography studies of proteins that provide high-resolution structures of proteins, including the locations of individual hydrogen atoms that have been exchanged for deuterium to make them particularly visible. Indeed, neutron crystallography can provide unique information on the chemistry occurring within biological macromolecules, such as enzymes, as recent studies on HIV-1 protease, an enzyme essential for the life-cycle of the HIV virus, illustrate.
Treating and stopping COVID-19
Proteases are like biological scissors that cleave polypeptide chains – the primary structure of proteins – at precise locations. If the cleavage is inhibited, for example, by appropriate anti-viral drugs, then so-called poly-proteins remain in their original state and the machinery of virus replication is blocked. For the treatment to be efficient this inhibition has to be robust—that is, the drug occupying the active site should be strongly bound, ideally to atoms in the main chain of the protease. This will increase the likelihood that treatments are effective in the long run, despite mutations of the enzyme, since mutations occur only within the side chains of the enzyme. Neutron research, therefore, provides essential input into the long-term development of pharmaceuticals. This role will be further enhanced in the context of advanced computer-aided drug development that will rely on an orchestrated combination of high-power computing, artificial intelligence and broad-band experimental data on structures.
Neutron crystallography data add supplementary structural information to X-ray data by providing key details regarding hydrogen atoms and protons, which are critical players in the binding of such drugs to their target enzyme through hydrogen bonding, and revealing important details of protein chemistry that help researchers decipher the exact enzyme catalytic pathway. In this way, neutron crystallography data can be hugely beneficial towards understanding how these enzymes function and the design of more effective medications to target them. For example, in the study of complexes between HIV-1 protease – the enzyme responsible for maturation of virus particles into infectious HIV virions – and drug molecules, neutrons can reveal hydrogen-bonding interactions that offer ways to enhance drug-binding and reduce drug-resistance of anti-retroviral therapies.
More than half of the SARS-CoV-2-related structures determined thus far are high-resolution X-ray structures of the virus’s main protease, with the majority of these bound to potential inhibitors. One of the main challenges for performing neutron crystallography is that larger crystals are required than for comparable X-ray crystallography studies, owing to the lower flux of neutron beams relative to X-ray beam intensities. Nevertheless, given the benefits provided by the visualisation of hydrogen-bonding networks for understanding drug-binding, scientists have been optimising crystallisation conditions for the growth of larger crystals, in combination with the production of fully deuterated protein in preparation for neutron crystallography experiments in the near future. Currently, teams at ORNL, ILL and the DEMAX facility in Sweden are growing crystals for SARS-CoV-2 investigations.
Proteases are, however, not the only proteins where neutron crystallography can provide essential information. For example, the spike protein (S-protein) of SARS-CoV-2 that is responsible for mediating the attachment and entry into human cells is of great relevance for developing therapeutic defence strategies against the virus. Here, neutron crystallography can potentially provide unique information about the specific domain of the S-protein where the virus binds to human cell receptors. Comparison of the structure of this region between different variations of coronavirus (SARS-CoV-2 and SARS-CoV) obtained using X-rays suggests small alterations to the amino-acid sequence may enhance the binding affinity of the S-protein to the human receptor hACE2, making SARS-CoV-2 more infectious. Neutron studies will provide further insight into this binding, which is crucial for the attachment of the virus. These experiments are scheduled to take place, e.g. at ILL and ORNL (and possibly MLZ), as soon as large enough crystals have been grown.
The big picture
Biological systems have a hierarchy of structures: starting from molecules that assemble into structures such as proteins; these form complexes which, as supramolecular arrangements like membranes, are the building blocks of cells. These are of course the building blocks of our bodies. Every part of this huge machinery is subject to continuous reorganisation. To understand the functioning, or in the case of a disease, the malfunctioning of a biological system, we therefore must get insight into the biological mechanism on all of these different length scales.

When it comes to studying the function of larger biological complexes such as assembled viruses, SANS becomes an important analytical tool. The technique’s capacity to distinguish specific regions (RNA, proteins and lipids) of the virus – thanks to advanced deuteration methods – enables researchers to map out the arrangement of the various components, contributing invaluable information to structural studies of SARS-CoV-2. While other analytical techniques provide the detailed atomic-
resolution structure of small biological assemblies, neutron scattering allows researchers to pan back to see the larger picture of full molecular complexes, at lower resolution. Neutron scattering is also uniquely suited to determining the structure of functional membrane proteins in physiological conditions. Neutron scattering will therefore make it possible to map out the structure of the complex formed by the S-protein and the hACE2 receptor.
Neutrons can penetrate deep into matter without damaging the samples
Last but not least, a full understanding of the virus’s life cycle requires the study of the interaction of the virus with the cell membrane, and the mechanism it uses to penetrate the host cell. SARS-CoV-2 is a virus, like HIV, that possesses a viral envelope composed of lipids, proteins and sugars. By providing information on its molecular structure and composition, the technique of neutron reflectometry – whereby highly collimated neutrons are incident on a flat surface and the intensity of reflected radiation is measured as a function of angle or neutron wavelength – helps to elucidate the precise mechanism the virus uses to penetrate the cell. Like in the case of SANS, the strength of neutron reflectometry relies on the fact that it provides a different contrast to X-rays, and that this contrast can be varied via deuteration allowing, for example, to distinguish a protein inserted into the membrane from the membrane itself. Regarding SARS-CoV-2, this implies that neutron reflectometry can in fact provide detailed structural information on the interaction of small protein fragments, so-called peptides, that mimic the S-protein and that are believed to be responsible for binding with the receptor of the host cell. Defining this mechanism, which is decisive for the infection, will be essential to controlling the virus and its potential future mutations in the long term.
Tool of choice
And we should not forget that viruses in their physiological environments are highly dynamic systems. Knowing how they move, deform and cluster is essential for optimising diagnostic and therapeutic treatments. Neutron spectroscopy, which is ideally suited to follow the motion of matter from small chemical groups to large macromolecular assemblies, is the tool of choice to provide this information.
The League of Advanced European Neutron Sources (CERN Courier May/June 2020 p49) has rapidly mobilised to conduct all relevant experiments. We are equally in close contact with our international partners, some of whom have, or are just in the process of, reopening their facilities. Scientists have to make sure that each research subject is provided with the best-suited analytical tool – in other words, those that have the samples will be given the necessary beam time. Neutron facilities are fast-adapting with special access channels to beam time having been implemented to allow the scientific community to respond without delay to the challenge posed by COVID-19.





