Water is well known for its astonishing range of unusual properties, and now Thomas Mattsson and Michael Desjarlais of Sandia National Laboratories in New Mexico have suggested yet another one. They found that water should have a metallic phase at temperatures of 4000 K and pressures of 100 Gpa, which are a good deal more accessible than earlier calculations had indicated.
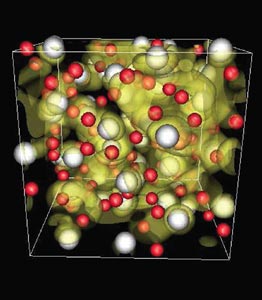
The two researchers used density functional theory to calculate from first principles the ionic and electronic conductivity of water across a temperature range of 2000–70,000 K and a density range of 1–3.7 g/cm3. Their calculations showed that as the pressure increases, molecular water turns into an ionic liquid, which at higher temperatures is electronically conducting, in particular above 4000 K and 100 GPa. This is in contrast to previous studies that indicated a transition to a metallic fluid above 7000 K and 250 GPa. Interestingly, this metallic phase is predicted to lie just next to insulating “superionic” ice, in which the oxygen atoms are locked into place but all the hydrogen atoms are free to move around.
Suitable conditions for metallic water should exist on the giant gas planets. In particular, the line of constant entropy (isentrope) on the planet Neptune is expected to lie in the region of temperature and pressure suggested by these studies for the metallic liquid phase.
Further reading
T R Mattsson and M P Desjarlais 2006 Phys. Rev. Lett. 97 017801.