Accelerator upgrades will help to meet the demand for strontium-82.
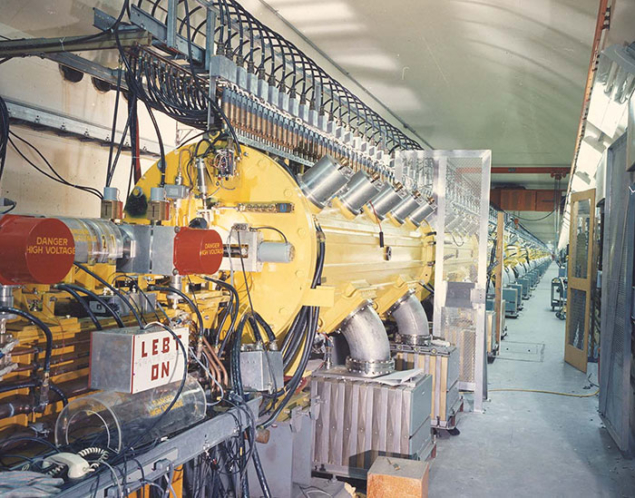
Image credit: BNL.
The mission of the US Department of Energy (DOE) isotope programme is to produce and distribute radioisotopes that are in short supply and in high demand for medical, industrial and environmental uses. The DOE programme also maintains the unique infrastructure of national laboratories across the country, one of which is Brookhaven National Laboratory’s medical radioisotope programme, MIRP. Although there are many small accelerators in the US that produce radioisotopes, the availability of proton energies up to 200 MeV from the Brookhaven Linac Isotope Producer (BLIP) is unique.
There is significant promise for treating a variety of diseases including metastatic cancer, viral and fungal infections and even HIV
Radioisotopes are of interest both for nuclear medicine and for diagnostic imaging and therapy. The most important aspect of Brookhaven’s isotope programme is the large-scale production and supply of clinical-grade strontium-82 (82Sr). Although 82Sr is not directly used in humans, its short-lived daughter product 82Rb is a potassium mimic and upon injection is rapidly taken up by viable cardiac tissue. It is therefore supplied to hospitals as a generator for positron emission tomography (PET) scans of the heart, where its short half-life (76 seconds) allows multiple scans to be performed and minimal doses delivered to the patient. At present, up to 350,000 patients per year in the US receive such PET scans, but demand is growing beyond capacity.
There is also significant promise for the utilisation of alpha emitters for treating a variety of diseases including metastatic cancer, viral and fungal infections and even HIV, for which the leading candidate is the alpha-emitter 225Ac. Thanks to a series of upgrades completed this year, Brookhaven is now in a position to boost production of both of these vital medical isotopes.
Protons on target
The BLIP was built in 1972 and was the world’s first facility to utilise high-energy, high-current protons for radioisotope production. It works by diverting the excess beam of Brookhaven’s 200 MeV proton linac to water-cooled target assemblies that contain specially engineered targets and degraders to allow optimal energy to be delivered to the targets. The use of higher-energy particles allows relatively thick targets to be irradiated, in which the large number of target nuclei compensate for the generally smaller reaction cross-sections compared to low-energy nuclear reactions.
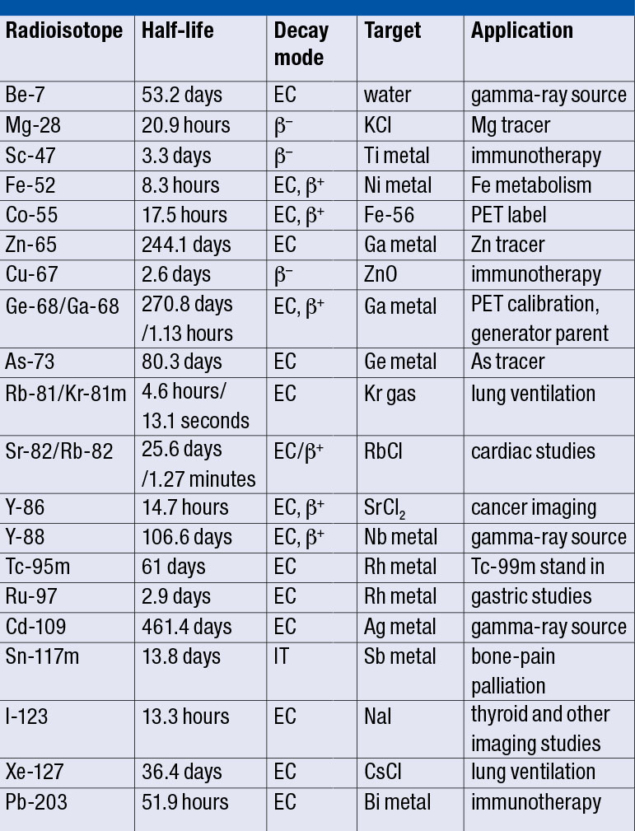
Although the maximum proton energy is 200 MeV, lower energies can be delivered by sequentially turning off the accelerating sections to achieve 66, 92, 117, 139, 160, 181 and 200 MeV beams. This is the only linac with such a capability, and its energy and intensity can be controlled on a pulse-by-pulse basis. As a result, the linac can simultaneously supply high-intensity pulses to the BLIP and a low-intensity polarised proton beam to the booster synchrotron for injection into the Alternating Gradient Synchrotron (AGS) and the Relativistic Heavy Ion Collider (RHIC) for Brookhaven’s nuclear-physics programme. This shared use allows for cost-effective operation. The BLIP design also enables bombardment of up to eight targets, offering the unique ability to produce multiple radioisotopes at the same time (see table). Target irradiations for radiation-damage studies are also performed, including for materials relevant to collimators used at the LHC and Fermilab.
The Gaussian beam profile of the linac results in very high power density in the target centre. Until recently, the intensity of the beam was limited to 115 μA to ensure the survival of the target. This year, however, a raster system was installed that allows the current on the target to be increased by allowing a more uniform deposition of the beam across the target. This system requires rapid cycling magnets and power supplies to continuously move the beam spot, and has been fully operational since January 2016.
Production of 82Sr is accomplished by irradiating a target comprising rubidium-chloride salt with 117 MeV protons, with the raster parameters driven by the thermal properties of the target. This demanded diagnostic devices in the BLIP beamline that enable the profile of the beam spot to be measured, both for initial device tuning and commissioning and for routine monitoring. These included a laser-profile monitor, beam-position monitor and plunging multi-wire devices. It was also necessary to build an interlock system to detect raster failure, because the target could be destroyed rapidly if the smaller-diameter beam spot stopped moving. The beam is moved in a circular pattern at a rate of 5 kHz with two different radii to create one large and one smaller circle. The radius values and the number of beam pulses for each radius can be programmed to optimise the beam distribution, allowing a five-fold reduction in peak power density.
Given the resulting increase in current from these upgrades, a parallel effort was required to increase the linac-beam intensity. This was accomplished by extending the present pulse length by approximately five per cent and optimising low-energy beam-transport parameters. These adjustments have now raised the maximum beam current to 173 μA, boosting radioisotope production by more than a third. After irradiation, all targets need to be chemically processed to purify the radioisotope of interest from target material and all other coproduced radioisotopes, which is carried out at Brookhaven’s dedicated target-processing laboratory.
Tri-lab effort
Among the highest-priority research efforts of the MIRP is to assess the feasibility of using an accelerator to produce the alpha emitter 225Ac. Alpha particles impart a high dose in a very short path length, which means that high doses to abnormal diseased tissues can be delivered while limiting the dose to normal tissues. Although there have been several promising preclinical and clinical trials of alpha emitters in the US and Europe, the 10 day half-life of 225Ac would enable targeted alpha radiotherapy using large proteins such as monoclonal antibodies and peptides for selective treatments of metastatic disease. 225Ac decays through multiple alpha emissions to 213Bi, which is an alpha emitter with a half-life of 46 minutes and can therefore be used with peptides and small molecules for rapid targeted alpha therapy.

Image credit: BNL.
Although 225Ac is the leading-candidate alpha emitter, vital research has been hindered by its very limited availability. To accelerate this development, a formal “Tri-Lab” collaboration has been established between BNL and two other DOE laboratories: Los Alamos National Laboratory (LANL) and Oak Ridge National Laboratory (ORNL). The aim is to evaluate the feasibility of irradiating thorium targets with high-energy proton beams to produce much larger quantities of 225Ac for medical applications. Because there is a direct correlation between beam intensity and radioisotope yields, the higher the intensity the higher the yield of these and other useful isotopes. So far, BNL and LANL have measured cross-sections, developed and irradiated relevant alpha-emitter targets for shipment to ORNL and other laboratories. These include several targets containing 225Ac-radioactivity up to 5.9 GBq and others for chemical and biological evaluation of both direct 225Ac use as well as use of a generator to provide the shorter-lived 213Bi. Similar irradiation methods are available at LANL and also TRIUMF in Canada.
Irradiation of thorium metal at high energy also creates copious fission products. This complicates the chemical purification but also creates an opportunity because some coproduced radiometals are of interest for other medical applications. The BNL group therefore plans to develop and evaluate methods to extract these from the irradiated-thorium target in a form suitable for use. In addition to 225Ac, the BNL programme is evaluating the future production of other radioisotopes that can be used as “theranostics”. This term refers to isotope pairs or even the same radioisotope that can be used for both imaging and therapeutic applications. Among the potentially attractive isotopes for this purpose that can be produced at BLIP are the beta- and gamma-emitters 186Re and 47Sc.
BNL has served as the birthplace for nuclear medicine from the 1950s, and saw the first use of high-intensity, high-power beams for radioisotope production. Under the guidance of the DOE isotope programme, the laboratory is using its unique accelerator facilities to develop and supply radioisotopes for imaging and therapy. Completed and future upgrades will enable large-scale production of alpha emitters and theranostics to meet presently unmet clinical need. These will enable personalised patient treatments and overall improvements in patient health and quality of life.





