A team of scientists from the Paul Scherrer Institute (PSI), CERN’s ISOLDE facility and the Institut Laue-Langevin (ILL) has published results from a preclinical study of new tumour-targeting radiopharmaceuticals based on the element terbium. The results demonstrate the potential of providing a new generation of radioisotopes with excellent properties for the diagnosis and treatment of cancer.
Radiopharmaceuticals in which a radioactive isotope is attached to a carrier that selectively delivers it to tumour cells are used in two main ways, for diagnosis and for treatment. Nuclear imaging for diagnostics involves either β+-emitting radioisotopes for positron-emission tomography (PET) or γ-emitting radioisotopes for use in single-photon-emission computed tomography (SPECT) and in planar imaging with gamma-cameras. By contrast, targeted radionuclide employs the short-range radiation (α-particles and electrons) emitted by radioisotopes to destroy cancer cells.
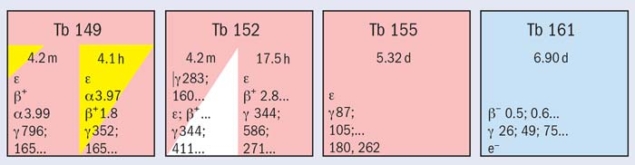
So-called “matched pairs” of diagnostic and therapeutic radioisotopes of the same chemical element are particularly useful because they allow the preparation of radiopharmaceuticals that are absorbed and distributed in identical ways in the body. Terbium is the only element in the periodic table to offer not just a pair but four clinically interesting radioisotopes with complementary nuclear-decay characteristics covering all of the options for nuclear medicine: 152Tb for PET, 155Tb for SPECT, 149Tb for α-particle therapy and 161Tb for therapy with electrons (β–, conversion and Auger electrons).
The team from the PSI, ILL and CERN has now made the first comprehensive preclinical study of this range of terbium radiopharmaceuticals. The neutron-deficient isotopes 149Tb, 152Tb and 155Tb were produced by 1.4 GeV proton-induced spallation in a tantalum target and separated with the ISOLDE online isotope separator at CERN. 161Tb was produced at the high-flux reactor of ILL and at the spallation neutron source SINQ at PSI. The isotopes were then purified using cation-exchange chromatography at PSI.
For this first in vivo proof-of-principle study the team developed a new delivery agent, which targets folate receptors in the body. These receptors are over-expressed in a variety of aggressive tumours, including ovarian and other gynaecological cancers as well as certain breast, renal, lung, colorectal and brain cancers, while their distribution in normal tissues and organs is highly limited. Folate vitamins have a rapid uptake in the body but they are also rapidly eliminated, so they do not remain long enough to reach all cancer cells. Hence, the team designed a new folate delivery agent called “cm09”, where folic acid is conjugated with an albumin-binding entity to prolong the circulation time in the blood.
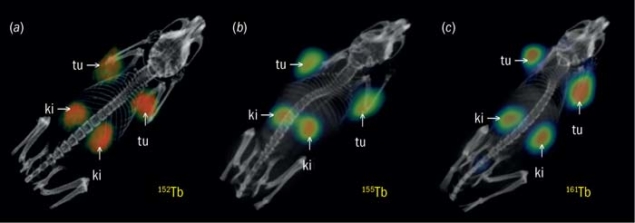
For the study, the terbium radioisotopes were combined with the cm09 and then administered to tumour-bearing mice. Excellent tumour-to-background ratios 24 hours after injection allowed tumour xenografts in mice to be seen using small-animal PET (152Tb-cm09) and small-animal SPECT (155Tb-cm09 and 161Tb-cm09). In vivo therapy experiments using 149Tb-cm09 (α-therapy) and 161Tb-cm09 (β-therapy) resulted in a marked delay in tumour growth or even complete remission, as well as a significant increased survival in treated animals compared with untreated controls.
Future progress in these promising diagnostic and treatment options depends crucially on the regular availability of the terbium isotopes, in particular of 149Tb. At present ISOLDE at CERN is the world’s only source of this isotope.
Further reading
C Müller et al. 2012 J. Nucl. Med. 53 1951.





