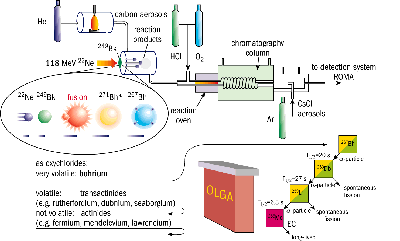
An international collaboration of radiochemists has carried out the first chemical study of the transuranic element bohrium (atomic number 107) using the Philips cyclotron at the Swiss Paul Scherrer Institute.
While the recent discovery of elements 114, 116 and 118 have grabbed the scientific headlines, these experiments do not yield any information about chemical properties. Such information is a pre-requisite for the classification of an element in the Periodic Table. From a chemist’s point of view, the Periodic Table ended with seaborgium, which has an atomic number of 106.
The bohrium experiment aimed to close the information gap, and investigate whether the element belongs to Group VII (which includes the elements rhenium and technetium). However, chemistry is by no means straightforward. Relativistic effects can strongly distort the electronic structure of elements and, in turn, lead to unexpected deviations in chemical properties compared with lighter homologues in the Periodic Table.
In the first experiment, performed in spring 1999 at the 88-inch cyclotron at the Lawrence Berkeley National Laboratory (LBNL), a new, long-lived isotope of bohrium, with a mass number of 267, was produced in the reaction between neon-22 ions and a berkelium-249 target. This new isotope was found to have a half-life of about 20 s, which is long enough for chemical investigation.
Previous investigations of heavy-element compounds that gave sufficiently high reaction rates and unambiguously identified themselves as members of a given group, showed that the most promising method of confirming bohrium as a Group VII element was by studying its oxychloride. In the case of Group VII elements, these molecules become volatile at much lower temperatures than those of the actinides (Group III) and the neighbouring transactinides (Groups IV-VI).
During a one-month period of beam time at PSI in September 1999, a 600 g/cm2 target of berkelium-249 was bombarded with 2 x 1012 neon-22 ions/s. The target material was provided by the US Department of Energy and prepared on thin beryllium foils by LBNL.
Using a gas transport system, the products were continuously injected into an on-line gas-chromatography apparatus (OLGA), which was capable of measuring the volatility of the pre-formed oxychlorides. Confirmation of the presence of bohrium, with single-atom sensitivity, was achieved using a rotating wheel multidetector analyser (ROMA). The analyser was equipped with solid-state detectors to register both the alpha-particle emission and spontaneous fission events, which are characteristic of the decay of such heavy nuclei.
Using a total of just six detected atoms, it was shown that bohrium indeed forms volatile oxychlorides at a temperature of 180 ºC, within the expected range of 200 ºC for Group VII elements. This, together with the registered alpha-decay chains, starting at bohrium-267 and passing through dubnium-263 (atomic number 105) and lawrencium-259 (103) to the long-lived mendelevium-255 (101), show that bohrium is an ordinary member of Group VII.





